Xenon-cryptophane complexes.
This section is related to the bio-sensor project and is the necessary upstream work for further developments in bio-sensing. We will first present our results on the complexation properties of xenon gas by cryptophanes in organic solution and then in aqueous media. We must also underline the discovery of a new cryptophane-111 optimized for xenon complexation that is still actively investigated.
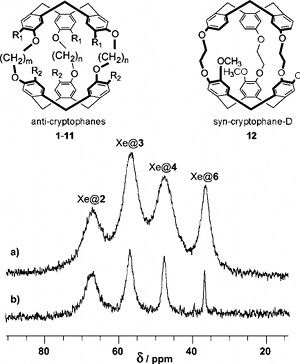 |
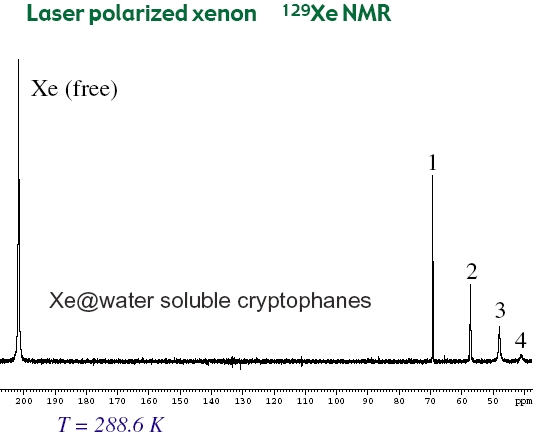
Cryptophanes form with xenon host-guest complexes that were analyzed by NMR using thermal or hyperpolarized noble gas in collaboration with the NMR group in CEA Saclay (P. Berthault). We have investigated 12 cryptophane hosts differing by their stereochemistry, cavity size and their substituents on the aromatic rings in order to extract information for the optimization of xenon-based biosensors (see below). Binding constants, dynamics of the host-guest exchange and relaxation of the 129Xe nucleus were examined. The results reported (J. Phys. Chem. A, 2008) showed that xenon is better accommodated in the smallest cryptophanes and that its long relaxation time inside the cavity showed that it is favorable for conservation of its hyperpolarization. However, very slow in-out exchange represents a limitation for future applicability for the biosensing approach. |
|
|
Cryptophanes bearing OCH2COOH groups in place of methoxy groups represent a class of xenon-carrier molecules soluble in water at biological pH. By using 1H and 129Xe NMR we have studied the structural and dynamics behaviors of these hosts as well as their interaction with xenon guest. Unprecedented high binding constants were found and make them excellent candidates for biosensing. Using xenon-cryptophane complexes in biological application (e.g. NMR imaging) needs to utilize laser-polarized 129Xe that increases the 129Xe NMR signal to noise ratio by at least a factor of 104. This has been performed in collaboration with the group of P. Berthault in CEA Saclay with whom we still collaborate for laser-polarized xenon NMR studies (J. Am. Chem. Soc., 2006). |
|
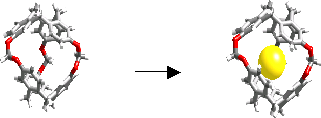
Recently, we reported the smallest cryptophane synthesized so far (cryptophane-111), which exhibits the highest binding constant to date K = 10000 M-1 at 293 K (in chloroform), for xenon encapsulation in organic solvent, to compare with the binding constant K = 3900 M-1 at 278 K determined for cryptophane-A in tetrachloroethane. Furthermore, a very slow decomplexation kinetics resulting in an extremely sharp, low-frequency shifted 129Xe NMR signal was observed (J. Am. Chem. Soc. 2007). Cryptophane-111 is actively studied to make it water-soluble and to develop new strategies for the synthesis of functionalized derivatives. This new cryptophane will be also used to complex other small gaseous guest like methane and hydrogen. |
 |