Recognition with Hemicryptophanes
This thematic deals with the recognition of guest molecules in the cavity of hemicryptophane hosts. Depending on the size and shape of the cage compound, zwitterions, ion pairs, ammoniums or carbohydrates can be recognized.
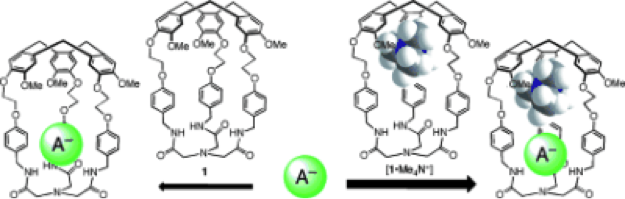
Cooperativity favors anion recognition in heteroditopic hemicryptophane complexes
Hemicryptophane selectively binds
taurine neurotransmitter in water
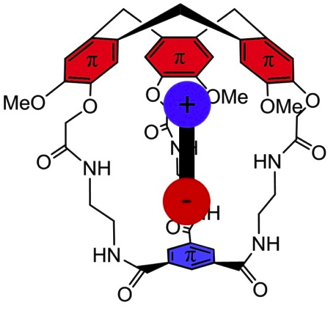
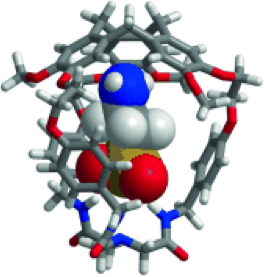
Brothers enemies anion-π and cation-π interactions act in a synergistic way when gathered in the molecular cavity of a hemicryptophane host to afford –170 kJ/mol efficient contributions in zwitterions recognition.
Persons involved: Oliver Perraud, Aline Schmitt, Alexandre Martinez, Jean-Pierre Dutasta
Related Publications:
1. “Gathering Cation-π and Anion-π Interactions for Zwitterions Recognition” O. Perraud, V. Robert, H. Gornitzka, A. Martinez, J. P. Dutasta Angew. Chem. Int. Ed. 2012, 51, 504-508.
2. “Hemicryptophane host as efficient primary alkylammonium ion receptor” O. Perraud, V. Robert, H. Gornitzka, A. Martinez, J. P. Dutasta Org. Bio. Chem. 2012, in press.
3” A Designed Cavity for Zwitterionic Species: Selective Recognition of Taurine in Aqueous Media” O. Perraud, V. Robert, A. Martinez, and J.-P. Dutasta Chem. Eur. J. 2011, in press, DOI: 10.1002/chem.201101522
5. “The Cooperative Effect in Ion-Pair Recognition by a Ditopic Hemicryptophane Host“ O. Perraud, V. Robert, A. Martinez, and J.-P. Dutasta, Chem. Eur. J. 2011, 17, 4177.
6. “Exclusive enantioselective recognition of glucopyranosides by inherently chiral hemicryptophanes” O. Perraud, A. Martinez, and J.-P. Dutasta, Chem. Comm., 2011, 47, 5861.



