Atrane Hemicryptophane-metal complexes
Our goal is to introduce a highly reactive site inside the cavity of a hemicryptophane host in order to perform catalysis in confined space. Thus, cage compounds bearing endohedral functionalities have been obtained and display outstanding properties: the confined space leads to an enhancement of the catalytic activity or to strong decrease of the rate of proton transfer in superbasic systems. Furthermore, these supramolecular objects have entered the field of molecular machine since they were able to control the directionality of the motion of atrane propellers.
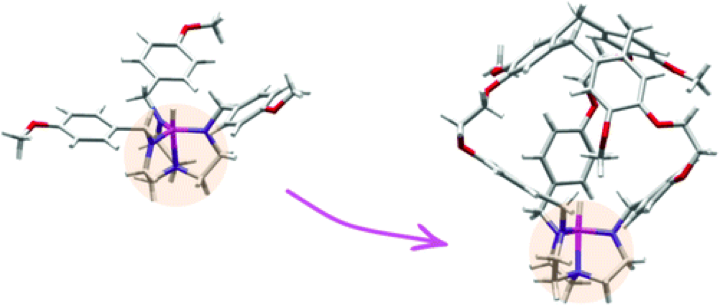
The properly designed host molecule and its protonated counterpart were synthesized to study how confinement can modify the stability and the reactivity of a Verkade’s superbase.
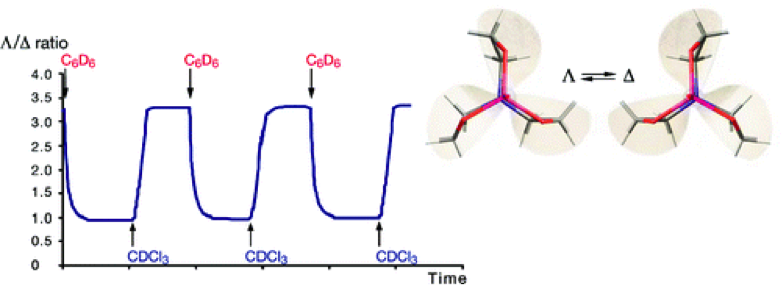
The ratio of the rates of the clockwise and anticlockwise tilting motions of the atrane structure shows that the solvent directs the rotational motion of the vanatrane moiety, so the propeller sense of the motion can be considered as unidirectional.
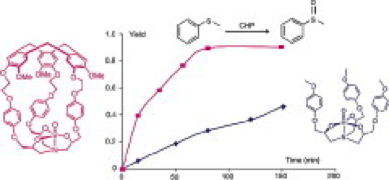
Hemicryptophane–oxidovanadium(V) complexes represent a new class of efficient supramolecular catalysts and show much higher catalytic performance in oxidation of sulfides in comparison with the model compound that lacks a molecular cavity.
Persons involved: Bastien Chatelet, Lionel Joucla, Alexandre Martinez, Jean-Pierre Dutasta
Related Publications:
1. “Encaging the Verkade’s Superbases: Thermodynamic and Kinetic Consequence“ P. Dimitrov Raytchev, A. Martinez, H. Gornitzka, and J.-P. Dutasta, J. Am. Chem. Soc., 2011, 133, 2157.
2. “Controlling helical chirality in atrane structures: solvent dependent chirality sense in hemicryptophane oxidovanadium(V) complexes.” A. Martinez, V. Robert, H. Gornitzka J.-P. Dutasta Chem. Eur. J., 2010, 16, 520.
3. “Reversible, Solvent-Induced Chirality Switch in Atrane Structure: Control of the Unidirectional Motion of the Molecular Propeller” A. Martinez, L. Guy, J.-P. Dutasta, J. Am. Chem. Soc. 2010, 16733.
4. “Hemicryptophane-oxidovanadium(V) complexes: lead of a new class of efficient supramolecular catalysts.” A. Martinez, J-P. Dutasta, J. Catal. 2009, 267, 188.



