Resolution of Hemicryptophanes
Here, we focus on the resolution of hemicryptophane, since their synthesis, most of times, afford racemic mixture. This allows us to report the efficient and enantioselective recognition of carbohydrates.
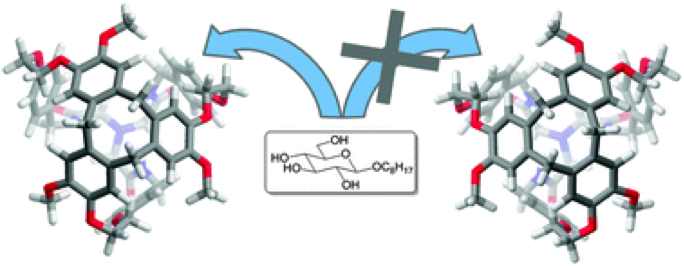
Inherently chiral hemicryptophanes were used to complex β- and α-glucoside derivatives with high diastereo- and enantio-selectivity.
Persons involved: Bastien Chatelet, Oliver Perraud, Aline Schmitt, Laure Guy, Alexandre Martinez, Jean-Pierre Dutasta
Related Publications:
1. “Resolution and Absolute Configuration Assignement of a Chiral Hemicryptophane Molecular Cage.” O. Perraud, P. Dimitrov-Raytchev, A. Martinez, J.-P. Dutasta, Chirality, 2010, 22, 885.
2. “absolute configuration and enantiodifferentiation of a hemicryptophane incorporating an azaphosphatrane moiety”E. Payet, P. Dimitrov-Raytchev, Bastien Chatelet, Laure Guy, J. Lacour, A. Martinez, J-P. Dutasta, en préparation.
3. “Exclusive enantioselective recognition of glucopyranosides by inherently chiral hemicryptophanes” O. Perraud, A. Martinez, and J.-P. Dutasta, Chem. Comm., 2011, 47, 5861.



