The cell is a level of biological organisation that has been poorly explored from an evolutionary perspective. How do cellular mechanisms evolve? What is the extent of possible solutions that have been retained across evolution to achieve basic cellular functions?
We are currently focusing on two main projects to address these questions. We are contributing to the emerging field of evolutionary cell biology.
1) Evolution of the mechanisms controlling spindle positioning in nematode embryos
Apparent conservation of phenotypes can reveal a diversity of underlying mechanisms. One need to describe this diversity to 1) understand the evolutionary dynamics of the system, 2) describe the system in its complexity, not relying on few model organism as reference species, 3) identify potential new mechanisms, that are hidden in laboratory species.

We are willing to address this question in the context of a cellular function, because very little is know about evolutionary cell biology. To this end, nematode embryos are great because they are big cells, their first cell division is very fast (15 minutes in C. elegans) and many subcellular events can be analyzed by simple DIC microscopy. Moreover, the one-cell embryo of the reference species C. elegans has been extensively studied both at the biophysical and molecular level, offering a fantastic framework to start comparative analysis. Finally, many nematode genomes are now sequenced or will be sequenced, allowing correlation between phenotypic and molecular evolution.
The first embryonic division of many nematode species is asymmetric, giving rise to two daughter cells of unequal size and fate. In C. elegans, this is achieved by the asymmetric positioning of the mitotic spindle along the polarity axis of the cell. During its asymmetric positioning, the spindle elongates and also undergoes typical transverse oscillations. These movements have been studied in details in C. elegans and reflect the action of mechanical forces acting on the spindle during mitosis. We have systematically recorded spindle movements during the asymmetric division of embryos from more than 40 nematode species. We have uncovered that very different combination of spindle movements ultimately lead to asymmetric cell division. Thus, mechanical optimization of the spindle differs between species.
Overall, an essential cellular function (asymmetric cell division) is maintained over the course of evolution while the underlying mechanisms that sustain it (asymmetric spindle positioning) change rapidly.
We are now exploring the species-specific mechanical optimization of the spindle to accomplish asymmetric cell division.
1.1) We will reveal evolutionary changes in the force balance in a subset of interesting species (experimental perturbations of embryos and biophysical measurements)
1.2) We will build a comprehensive physical model of force balance in the spindle, based on the effective description of native embryos. The model will constitute a powerful tool to define the range of solutions that the system can explore while maintaining a constant output.
1.3) We will identify molecular changes that are responsible for different spindle motion (analysis of publicly available genomes, comparative transcriptomic, etc.)
2) Cellular innovations at the origin of new reproductive modes in nematodes
Non-parasitic nematodes from the Rhabitidae family show a large diversity of reproductive modes.
We are exploring the cellular modifications that have allowed the emergence of new reproductive strategies. While the sperm centrosomes provide the first polarity cue of the C. elegans zygote, parthenogenetic species must polarize independently of this cue. In these species, centrosomes are not provided paternally, also raising the question of their origin, etc.
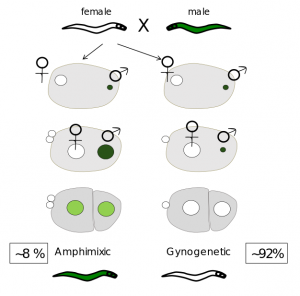 We are currently focusing on pseudogamous species of the Mesorhabditis genus. In this group, only 10% of males arefound. However, males are necessary to fertilize all the oocytes of females.
We are currently focusing on pseudogamous species of the Mesorhabditis genus. In this group, only 10% of males arefound. However, males are necessary to fertilize all the oocytes of females.
In one small category of embryos called « amphimixic », the sperm DNA decondenses and participates, together with the female haploid genome, to the formation of the zygote. In most embryos however, the sperm DNA is not used after fertilization, and the embryo develops only from the female DNA (« gynogenetic » embryos). In this case, the female meiosis is incomplete and despite the absence of the male DNA ,embryos remain diploid.
Strickingly, while gynogenetic embryos give rise to only females, we found that amphimixic embryos give only rise to males. Thus, the production of amphimixic embryos allows the maintenance of few males in the specis.
What makes this system unique, is that females systematically produce males who do not passed on their genes to females, and thus do not participate to the genetic diversity of the offspring.
Overall, stochasticity in the progression of the female meiosis, combined with a control of the paternal DNA has allowed the emergence of a unique and original mode of reproduction.
2.1) We are exploring the cellular basis of this innovation.
2.2) We are also exploring the consequences on the population genetic structure of these pseudogamous species.
