BioMath
Like many contemporary scientific fields, molecular and cellular biology raise new mathematical and computational challenges that are inherent to the massive production of large, high-resolution datasets that are complex and high-dimensional. This ever growing complexity requires the development of cutting-edge interdisciplinary research. The LBMC has been very active in the field, thanks to the development of collaborations with mathematicians, computer scientists and statisticians, who are now given the opportunity to develop their own methodological research within the laboratory to be at the core of current challenges in computational biology.
Systems biology is one major research axis in the LBMC. It aims at achieving the quantitative understanding of dynamic biological processes, through the use of mathematical and statistical analyses to integrate biological data, in order to develop predictive models of biological behaviours. In this respect we will develop our research along two methodological lines: Dynamical Modelling and AI & Machine Learning.
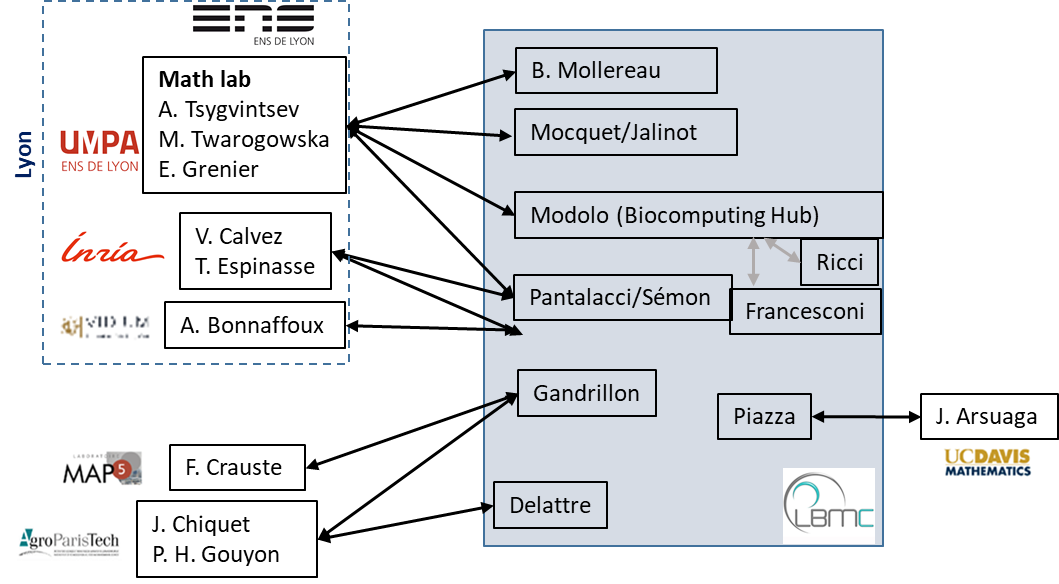
Overview of current interactions between LBMC teams and mathematicians
The Math-Info-Bio environment of the LBMC is further described here.
Dynamical modeling
Gene-centered approaches, as commonly conducted during the past decades, still present a useful way to focus experimental power and scientific reasoning on specific mechanisms, pathways and/or regulations. However, these must be complemented by a wider perspective that integrates the complexity of their biological context. Reciprocally, seeking a fine-scale understanding of cells in their entire complexity is not realistic, and often not necessary.
The challenge ahead is therefore to extract general principles, and possibly simple rules that govern the properties of living cells, while taking into account the multiplicity and complexity of their molecules. This can be done at a theoretical level, which is useful to develop novel concepts. Nonetheless, all theories need to be confronted with the reality of biological systems. To engage in such a practice, one therefore needs:
- Experimental data that is quantitative, and either focused (to offer specificity), or large enough to take into account the numerous molecules involved. Ideally these data will be precise in both space and time.
- Computational or mathematical models that can represent and study the collective and dynamic properties of relevant entities (molecules, cells...).
- Sustained interactions between model-based predictions and experiments to produce robust and rigorous conclusions.
The development of such computational or mathematical models is being pursued by several research groups at the LBMC as described below.
Systems Biology of Decision Making - O. Gandrillon
The inference of dynamical gene regulatory networks (GRNs) is a long standing theoretical and computational challenge that we tackle with very original approaches like optimal transport and stochastic processes. Once the GRNs have been inferred another challenge is to connect the cellular behavior to the behavior of the inner GRNs thanks to a dynamical PDE model incorporating molecular variables, or multiscale agent-based models.
This line of research will be developed through the Mémoire ANR project that involves collaboration with the Dracula team (INRIA), the ICL team (CIRI), Fabien Crauste (Université de Paris) and two private companies, Altrabio and Vidium.
Physical Biology of Chromatin - D. Jost
Epigenetic markers are tightly coupled to gene expression and cell lineage. Our group studies:
- The spreading of epigenetic marks coupled to three dimensional structure of the genome using mechanistic dynamical models, with a focus on silencing marks and DNA repair.
- The relationship between origins of replication and epigenetic landscape using mechanistic single cell models to reproduce experimental characterisation of the cell such as Mean Replication Timing using epigenetic marks, in collaboration with Benjamin Audit (ENS de Lyon) and Olivier Hyrien (ENS Paris).
Comparative and Integrative Genomics of Organ Development - S. Pantalacci / M. Semon
In the team, we focus on the temporal dynamics of developmental mechanisms as a key determinant of their outcome. In collaboration with Monika Twarogowska (UMPA, ENS de Lyon) and Vincent Calvez (Institut Camille Jordan, Univ Lyon 1), we have embedded a classical Turing model in an original framework and shown this was sufficient to recapitulate the complex dynamics of tooth patterning and predict its perturbation. We will now infer dynamical GRN for this patterning mechanism and study both conceptually and experimentally its variational properties throughout evolution.
Genome mechanics - A. Piazza
Our work aims at understanding the interplay between the spatial organization of the genome and the DNA repair process by homologous recombination. In particular, we focus on the homology search step, in which the broken DNA molecule samples genomic DNA for a homologous match. The stringency of this dynamic process promotes genomic stability, and involves multiple layers of control, at the molecular and chromosomal levels. To gain insights into this rate-limiting step of the repair process we develop various quantitative or semi-quantitative assays that provide kinetics information on the repair process, coupled with Hi-C to determine the averaged pair-wise dsDNA contact frequency genome-wide.
In collaboration with the laboratory of Javier Arsuaga (Department of Mathematics, UC Davis), we attempt to mathematically model the repair process in order to derive the quantitative contributions of multiple nucleoprotein factors involved in the search process, at the local level (e.g. recombination proteins) and chromosomal level (e.g. chromatin folding by cohesins).
Apoptosis and Neurogenetics - B. Mollereau
Most cancer treatments, such as chemotherapy or radiation-based therapy, eliminate cancer cells by inducing apoptosis, which is the most well studied form of programmed cell death. Although these treatments proved relatively successful for several cancers, malignant cells often develop resistance to apoptosis leading to the reduction or loss of treatment efficiency. There is thus an urgent need for alternative treatment for cancer therapy. Our consortium has found that programmed necrosis, which has been neglected for many years, is a potent inducer of cell death in cells that are resistant to apoptosis.
Previous results obtained by our consortium revealed an intriguing finding: blocking the activation of caspases, the molecular scissors that actually demolish the cell during apoptosis, enhances programmed necrosis in both fly retina cells and mice testis. Corroborated with literature reports suggesting that cancers could use non-lethal caspase activation in their own advantage, we propose and will investigate whether caspases could in fact block programmed necrosis in several malignancies such as melanoma, glioblastoma, lung and pancreatic cancers.
To understand the switch from apoptosis to necrosis in cancer cells, we will characterize the dynamic of the inhibition of necrosis by caspases by performing live imaging and mathematical modelling with Emmanuel Grenier (UMPA, ENS de Lyon).
RNA metabolism in immunity and infection (RMI2) - E. Ricci
In the team, we are interested in studying the dynamics of messenger RNA (mRNA) translation by ribosomes and its cross-talk with other cellular processes such as mRNA degradation. mRNA translation is a tightly regulated process in which ribosomes decode the information contained in the open reading frame (ORF) of mRNAs into a polypeptide sequence. Ribosome recruitment onto the mRNA and its translocation across the ORF are not a uniform process. Various obstacles can affect the progression of ribosomes and modify their decoding speed leading to conflicting situations such as ribosome stalling or collisions that can induce the degradation of the mRNA and the nascent polypeptide.
We use ribosome profiling coupled to next-generation sequencing to map the position of ribosomes on the coding sequence of all expressed mRNAs under regular conditions and after viral infection or immune cell activation. Using this data we then try to develop ODE-based mathematical models to explain ribosome progression across open reading frames based on transcript cis-acting features such as codon usage, GC content and length of untranslated regions.
Genetic complexity of living systems - G. Yvert
Genetics - the science that links phenotypes to genotypes - is rarely adapted to the highly-dynamic nature of living systems. For example, a classical approach is to introduce a mutation in a model organism and then examine the resulting phenotypic change. This can demonstrate the link but rarely its mode of action: how is this mutation causing this effect?
We are developing novel methods and concepts to understand how mutations modify the dynamics of molecular regulations. For example, by coupling experiments with mathematical modelling of gene regulations, we were able to delineate an intermediate layer of the genotype-phenotype association: specific model parameters had to be re-adjusted in response to a mutation, highlighting the molecular path by which this mutation operated.
Another approach is to study how natural selection and evolution shapes the adaptation of organisms to dynamic environments, which indicates the mutations that modify dynamic regulations in a beneficial way. We are also implementing optogenetic technologies enabling genetic perturbations with extreme spatiotemporal precision. This allows us to monitor the dynamic effects of mutations in real time. Coupling such experiments with kinetic models of stochastic processes can reveal how cells and organisms adapt - or not - to de novo mutations.
AI & Machine Learning
Conventional data analysis methods are largely designed for low-dimensional datasets and need to be adapted to the high dimensional / complexity setting. Consequently, new machine learning methods need to be developed to represent, analyse, summarize, integrate and exploit the full potential of single cell genomics data.
Indeed, recent methodological development relying on the development of single cell technologies give us the ability to measure, at the level of individual cells, genome-wide features such as DNA, RNA, chromatin states, proteins or metabolites. The use of these techniques allows us to study cell-to-cell variability within a biological sample and investigate new questions out of reach for classical genomics.
This complexity has reached an unprecedented level of complexity and heterogeneity with the new developments of spatial transcriptomics that connects molecular data to cellular localization of high dimensional features. All these challenge require the developments of complex machine learning methodologies based on non parametric and multiscale methods, that we will develop in the coming years.
This research line is currently being developed in the LBMC groups described below.
Systems Biology of Decision Making - O. Gandrillon
Investigating cell-to-cell variability is fundamental to better understand differentiation, as it reflects the intrinsic stochastic molecular processes and provides information on the underlying molecular networks at play. This line of research will be developed through the SingleStatOmics ANR project that gathers a national interdisciplinary consortium in machine learning / AI dedicated to single cell genomics, with experts in machine learning, optimal transport and statistics, both from the academic (Bertrand Michel, EC Nantes, Filippo Santambrogio, UCBL, Julien Chiquet, INRAE) and the private sector (Jean-Philippe Vert, GoogleBrain).
Physical Biology of Chromatin - D. Jost
To study the replication process at the single cell level, we developed a deep-learning based algorithm to decode the electrical current signals that output from nanopore sequencing into a DNA sequence with modified nucleotides. These nucleotides are incorporated in the cell during the replication process, allowing to monitor, genome-wide in yeast, the direction and speed of the replication forks at the single cell level. We are currently developing a method to monitor the timing of replication at the single cell level, in collaboration with Benjamin Audit (ENS de Lyon) and Olivier Hyrien (ENS Paris).
Quantitative regulatory genomics - M. Francesconi
In Francesconi’s team we are interested in understanding genetic and non-genetic sources of phenotypic variability. We focus on genome-wide gene expression dynamics – both at single-cell and single-individual level - as a multidimensional intermediate phenotype to infer information higher level phenotypes such as cellular states developmental stage. For example, using big data integration and machine learning approaches we recently developed a computational method to accurately infer developmental age of single individual expression profiles which we are now extending to single cell expression.
Post-transcriptional Regulation in Infection and Oncogenesis - Jalinot/Mocquet
We have developed an experimental approach aiming at identifying small chemical compounds prone to induce specifically proteolytic degradation of a given protein. The method is based on the analysis of the ratio between the fluorescences of a hybrid protein associating a protein of interest to a first fluorescent protein and a second control fluorescent protein. The measure is performed by flow cytometry analysis of cells expressing the two proteins. As the effect exerted by unoptimized compound can be faint, we have initiated a collaboration with Alexei Tsygvintsev (UMPA, ENS de Lyon) who develops artificial intelligence approaches for the analysis of experimental biology data to see if elaboration of an AI method using either graphical or numerical data sets can help to spot interesting candidates in the first screening round.
Comparative and Integrative Genomics of Organ Development - S.Pantalacci/M. Semon
In collaboration with mathematician Laurent Guéguen (LBBE, Univ Lyon 1) and biostatistician Marie-Laure Delignette-Muller (VetAgro-Sup, Univ Lyon 1) we model quantitative data, including RNA-seq data and morphological measurements, to extract temporal dynamics and compare them across species.
Scientific environment / Partners
The ENS-Lyon campus has long been involved in the developments of math-info-bio interactions, with the Complex Systems Institute (IXXI), that promotes modeling research in applied science, with a special focus on AI challenges in Systems Biology for the 2021-2026 mandate.
In particular, systems biology has been very active in Lyon, especially thanks to the Lyon federation for Systems Biology (BioSyl), and to the structuring effect of INRIA groups in the Lyon Campus (Virtual plant, Dracula, Numed, Bamboo, Beagle).
In addition, the Scidolyse consortium has emerged, dedicated to research in machine learning in Lyon Saint Etienne, with founding members being part of the UMPA, LIP and LBMC labs (among others).
The LBMC has also long been dedicated to interdisciplinary research (bio - math - physics) by developing strong connexions with INRIA and the Camille Jordan Institute (math lab, UCBL).
The recent funding of the Spatial-Cell-Id Equipex project will be a superb occasion to foster interaction between the LBMC and the Spatial-Cell-Id core units (IGFL, RDP ad SBRI).