Characterize the NMD pathway and its deregulation by viral factors (Vincent Mocquet- projet 1)
The NMD is a quality control pathway leading to the degradation of RNA that harbor ribosomes considered by the cell to have prematurely stopped. Besides PTC-containing RNAs, misreading of normal stop codon for a PTC due to various regulation events (presence of a 5’uORF, long 3’UTR sequences etc.) can also trigger NMD. Therefore NMD leads to the physiological regulation of non-mutated mRNAs, such as those involved in hematopoietic cell differentiation, the maintenance of chromosome structure and function as well as viral RNA. The dysregulation of the NMD pathway would then constitute a great threat for the stability of genetic information (at least at the RNA level) and, in turn, for the homeostasis of normal gene expression and cell behavior. NMD also emerges as a new antiviral process (Mocquet et al AIDS Res and Hum Retrov, 2015)
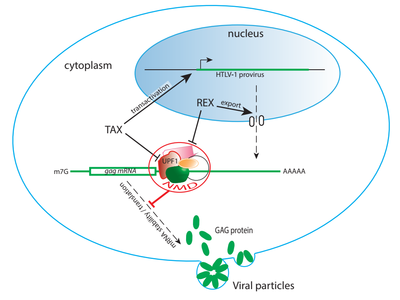
From discovery by C. Morris of the role of INT6 in the NMD pathway, we showed that HTLV-1 Tax inhibits this mRNA surveillance pathway by interacting with both INT6 and the NMD core factor UPF1 (Mocquet et al. JVI, 2012). By this action the virus favors the stabilization of its own viral mRNAs as well as specific cellular mRNAs. We are now analysing with care the impact of the Tax / UPF1 interaction on the enzymatic activities of UPF1 (Fiorini et al. Nat Comm, 2018) and on the host post-transcriptional regulation. Morevoer we are currently studying the necessity of NMD inhibtion for viral replication and its role in the emergence of associated pathologies.
Additional projects are focusing on a Rex dependent NMD inhibition.