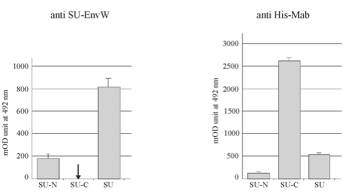
Env
ERVWE1 interaction with DC-SIGN.
Binding of the SU-N, SU-C and SU
proteins to oligomeric DC-SIGN was tested by using a cell-ELISA approach
described in [Erdile LF et al., 2001 ; Kataoka HN et al., 2000] and adapted to
DC-SIGN. Arrow indicates the inability to detect the binding of SU-Cprotein to
DC-SIGN molecules due to the absence of ant-SU-EnvW epitope onto SU-C protein.
M eans +/- standard deviation ; n=5.
Stable
DC-SIGN-expressing HeLa cells or parental HeLa cells were seeded in 96-well
plates at 4 x 104 cells per well. The cells were left to adhere overnight,
rinsed once and fixed by 4% paraformaldehyde in adhesion buffer (20 mM TRIS HCl
pH8, 450 mM NaCl, 2 mM MgCl2 and 1 mM CaCl2, adapted from [Geijtenbeek TB et
al., 2000]) for at least 15 min. After saturation, supernatants were added and
incubated for 1h30. Cells were saturated for one hour with 10% nonfat dry milk
in adhesion buffer and rinsed three times with wash buffer (adhesion buffer
containing 0.1% Tween 20). The detection was carried out using an anti-His-Mab
at dilution of 1:1,000 in adhesion buffer supplemented with 1% BSA. Following 1
hour of incubation at 37¡C, the plates were rinsed three times with wash
buffer, and 1 µg/ml of horseradish peroxydase-conjugated antibody was added to
each well and incubated at 37¡C for 1 hour. The plates were washed three times
with wash buffer and 100 µl of a mixture of H2O2 and o-phenylenediamine (Sigma
Aldrich) was added for colorimetric detection. The plates were left for 10 min
at room temperature in the dark and reaction was then stopped by adding 100 µl
of 1M H2SO4. Absorbance was measured at 492 nm. To take into account the
non-specific binding, assays were both carried out on stable DC-SIGN expressing
HeLa cells and on parental HeLa cells.
Erdile,
L. F., D. Smith, and D. Berd. 2001. Whole cell ELISA for detection of tumor
antigen expression in tumor samples. J Immunol Methods 258:47-53.
Kataoka,
H., N. Kume, S. Miyamoto, M. Minami, T. Murase, T. Sawamura, T. Masaki, N.
Hashimoto, and T. Kita. 2000. Biosynthesis and post-translational processing of
lectin-like oxidized low density lipoprotein receptor-1 (LOX-1). N-linked
glycosylation affects cell-surface expression and ligand binding. J Biol Chem
275:6573-9)
Geijtenbeek,
T. B., R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, G. J. Adema, Y. van
Kooyk, and C. G. Figdor. 2000. Identification of DC-SIGN, a novel dendritic
cell-specific ICAM-3 receptor that supports primary immune responses. Cell
100:575-85)
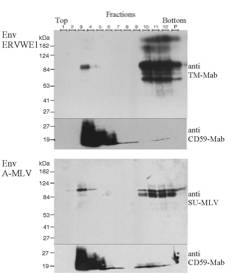
ERVWE1 is associated with detergent-resistant membranes (DRMs):
293T cells
were transfected with wild-type HERV-W plasmid or with amphotropic MLV envelope
expressor plasmid (phCMV-A). 36 hours later, cells were washed in PBS and lysed
in 1ml 1%Triton X-100 TNE buffer (150mM NaCl; 25mM Tris pH7.5 ; 1mM EDTA
containing Complete-Mini protease inhibitor cocktail, Roche) 30 min on ice.
Subsequently, lysates were mixed with 2ml of 45% sucrose (wt/vol in TNE) and
placed in SW41 centrifuge tube (Beckman). Samples were overlaid with 6ml of 30%
sucrose followed by 2.5ml 5% sucrose and spun 22 hours 38,000 rpm at 4¡C. The
gradient was collected in 1ml steps from the top to the bottom (1 to 12) and
the pellet was collected in 1ml TNE and sonicated (P). Aliquots of 30µl of each
fraction were analyzed by 9% polyacrylamide gel under non-reducing conditions.
Blots were probed with anti-TM-Mab for Env ERVWE1 at dilution of 1:5,000 and
with anti-SU-MLV for EnvA-MLV (Quality Biotech) at dilution of 1:2,000. DRMs
were labelled with a monoclonal anti-CD59 (BD PharMingen) at dilution of
1:1,000. The bulk of detergent-insoluble material is concentrated in fractions
3 and 4, whereas soluble material ends up in fractions 10 to 12.