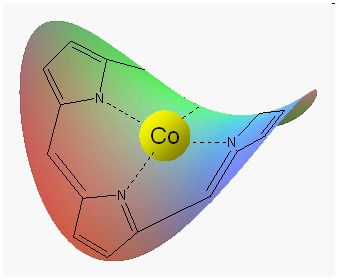
2 CO on Co and Fe porphine
PRESS RELEASE
A pair of CO in the saddle on porphyrins of cobalt and iron
Unexpected interactions between molecules similar to those involved in the mechanism of respiration were revealed by microscopy (STM) and confirmed by modelisation. This work, a collaboration between researchers from CNRS (ENS-Lyon), TUM, Munich and Barcelona CSIC-ICN, have shown that two molecules of carbon monoxide, and not just one, could bind to ions cobalt or iron, on the same face of a porphyrin core. It has been published in Nature Chemistry in 2011.
|
Many metalloporphyrins are involved in plenty of biological processes. They look like horse saddles with a metal ion in the center (Fig. 1). Such a center can bind a molecule of gas such as oxygen or carbon monoxide. Among the abundance of studies dedicated to these structures, the binding of two molecules of gas on the same side of the saddle has never been observed. This is done with cobalt and iron, two essential metals in biology. Moreover, the gas molecule does not bind to the central ion, but fits between this ion and a neighboring nitrogen atom. |
Figure 1 |
The study was possible by depositing a cobalt porphyrin or iron on a metal surface of silver or copper, allowing STM analysis, then exposing the sample to carbon monoxide. Microscopic images (Fig. 2) show, unambiguously, two options for fixing CO. The first corresponds to the binding of two CO. This is the most stable mode which reveals two distinct sites. The second is the establishment of a single molecule, fluctuating between the two sites.
Marie-Laure Bocquet (ENS Lyon & CNRS, Lyon) was able to confirm the interpretation of theses observations by ab initio modelisation of this system. She not only confirmed the presence of two sites, but also showed that cobalt was only bound to two of four binding nitrogen sites that hold the metal ion in the center of the porphyrin. She also quantified certain distances, especially between the structure and its support, and was able to specify the nature of interactions between different components of the structure.
|
The STM images (Fig. 2) confirms: |
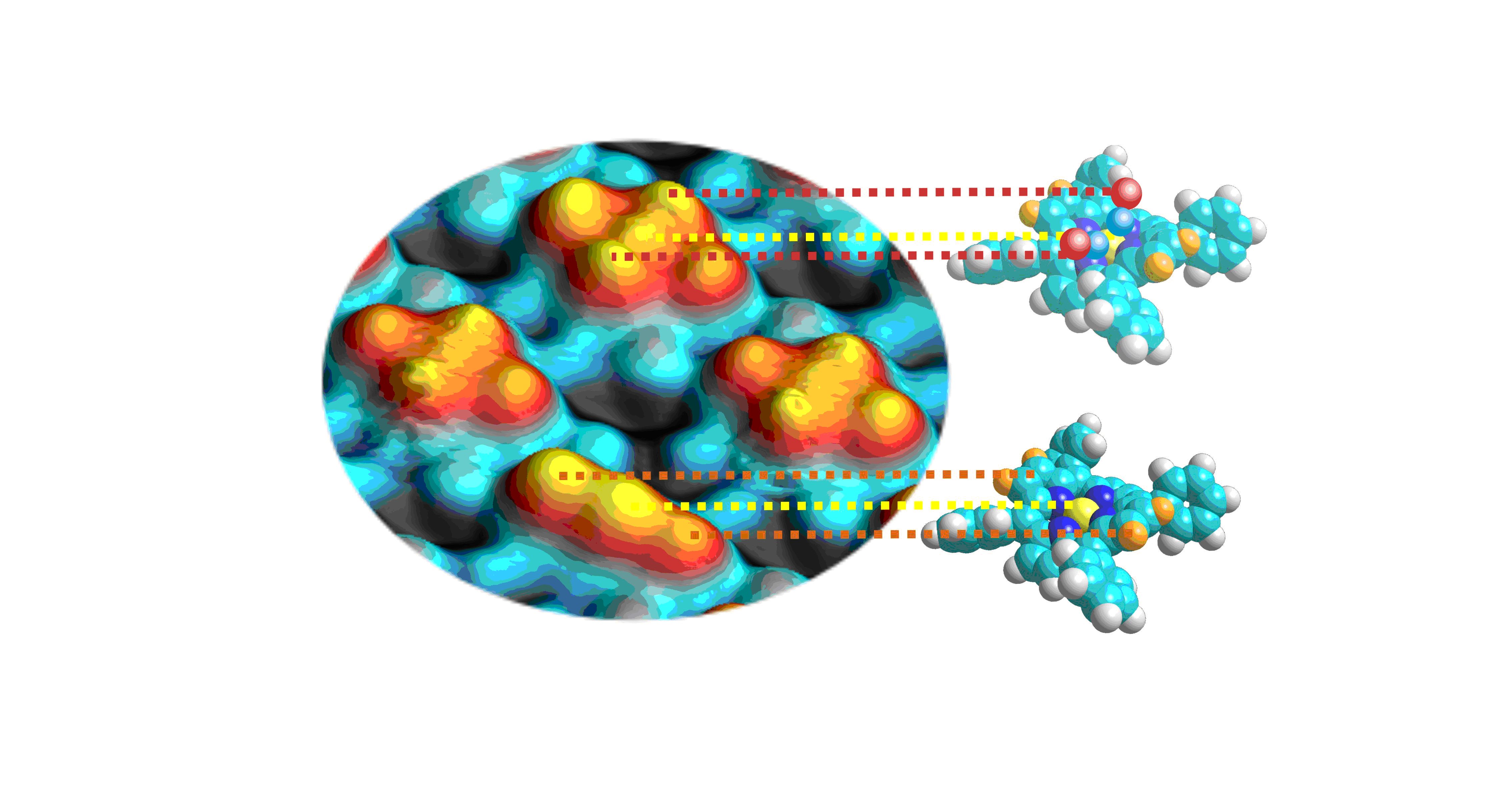
figure 2 |
Such a discovery opens up prospects for interpreting the interaction of many biological systems such as breathing mechanisms.
Figure 2 caption:
The blue spheres are the silver atoms of the absorbing material; the threefold yellow protrusion is the porphyrin that did not fix CO, with the cobalt ion in the center, the quintuple smooth yellow protrusion is that the porphyrin molecules has set two CO, and the two wrinkled quintuple protrusions are two porphyrins which have each set a single CO molecule. The distance between two neighboring protrusion is about 0.25 nm
Learn more
- Cis-dicarbonyl cobalt and iron binding at porphyrins with saddle-shape conformation. Knud Seufert, Marie-Laure Bocquet, Willi Auwärter, Alexander Weber-Bargioni, Joachim Reichert, Nicolás Lorente and Johannes V. Barth. Nature Chemistry, 3, 114-119, (2011).
- La recherche Mai 2011, n°452, p.15.