Dr. Natacha GILLET
| When |
Mar 21, 2019 à 10:30 AM |
|---|---|
| Where |
Salle André Collet |
| Contact |
Elise DUMONT |
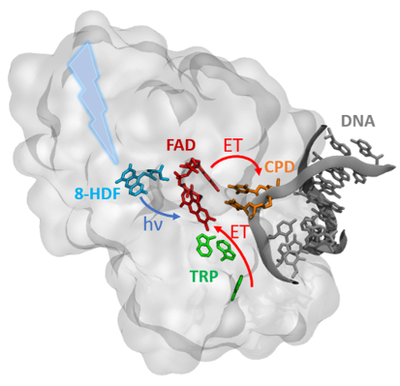
Evolution in the cryptochrome-photolyase family: a computational point of view
Cryptochromes and photolyases form an ubiquitous flavoprotein family involved as photoreceptor in regulation of circadian cycle, magnetoreception and photorepair of UV-damaged DNA. Despite these very different functions, they share similar features such as the presence of a buried FAD cofactor, photoactivated by a second chromophore, and a chain of aromatic residues, which is able to trigger photoreduction of the excited flavin. Some structural changes, highlighted by mutagenesis studies, manage the specificic role of each protein by controlling the FAD redox state or the DNA binding pocket.
We are interesting in different aspect of the structure-function relationship in this protein family. First, we used an efficient QM/MM approach to model the fast charge transfer occurring after FAD protonation. We studied different associations of aromatic residues with regard to evolution among the photolyase family. We also performed QM/MM biased simulation of the protonation of semi reduced FAD in plant cryptochrome (about microsecond timescale) and E. coli photolyase (second timescale), with respect to the active form of FAD in each protein. We gave some molecular explanation of such a timescale difference and of the inefficiencies of mutations in E. coli photolyase to increase the protonation rate. Finally, we were interested in the DNA-protein interaction in PhrB photolyase, which belongs to a phylogenetically ancient group. In this latter, divalent cations are essential for DNA-repair whereas their concentration has no impact on the function of more ‘recent’ photolyases. Our classical simulations provided structures explaining this behaviour, consistently with mutagenesis studies. Our multiscale theoretical approaches have provided valuable information to better understand the evolution of the cryptochrome-photolyase family.