Research projects
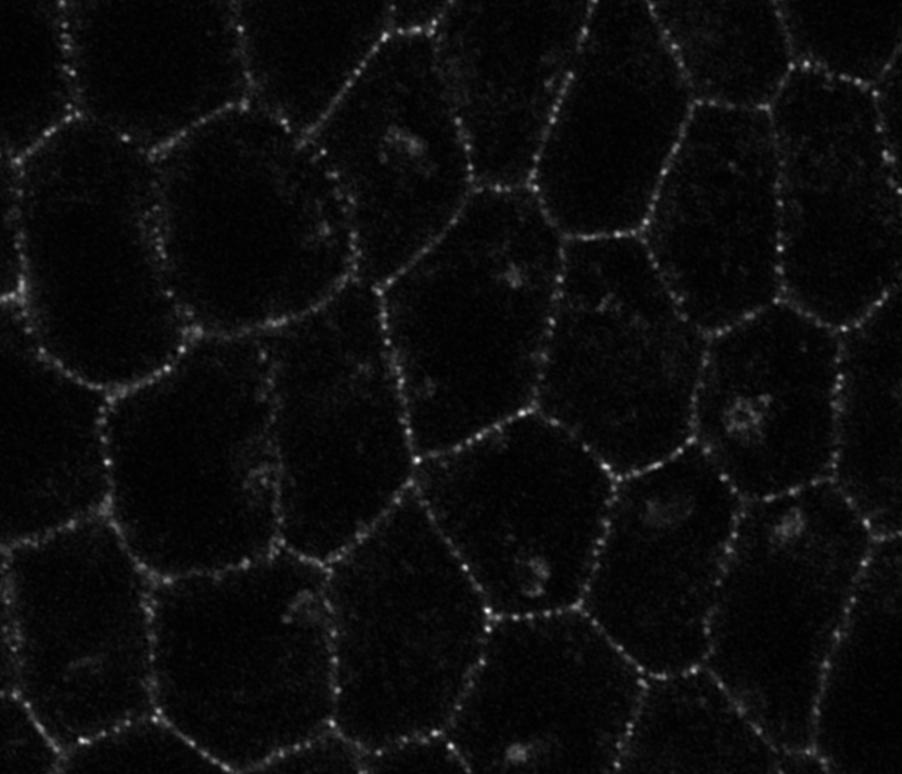
Epithelial cell shape depends on an internal balance between the actomyosin cytoskeleton, the Adherens Junctions and the Focal Adhesions. How these internal cell shape effectors are impacted by compressive forces generated by adjacent growing tissues is unknown. To tackle this question, we use the Drosophila ovarian follicle, in which cuboidal epithelial cells are apically compressed by germline cytoplasmic pressure, while simultaneously being basally constrained by a basement membrane. We previously demonstrated that these two surrounding tissues mechanically drives cuboidal cells to become either squamous or columnar. Our aim is to determine the mechanisms by which compressive forces (germline cytoplasmic pressure and basement membrane stiffness) modify cell shape effectors (the cytoskeleton and adhesion proteins) to allow cell morphogenesis.
To investigate this, we perform experiments in both WT and mutant conditions where the mechanical properties of the germline or the BM are altered either positively or negatively. We first use 3D reconstructions and in-vivo live imaging to quantify cell shape changes and define the sequence of remodelling events. Second, we characterise the differential subcellular localisation of E-Cad, integrin, actin and myosin and measure their dynamics through confocal and super-resolution microscopy. Third, because the activities of E-Cadherin and Integrin depend on their low-level organisation into nano-clusters, we perform homo-FRET experiments to obtain a detailed description of the nano-scale organisation of each subcellular accumulation (such as monomers or cis-oligomers). Fourth, we determine the mechanical properties of cuboidal, squamous and columnar cells by performing micro-compression, laser ablation and force inference measurements. Finally, we employ genetic and optogenetic tools to explore whether myosin activity differentially impacts adhesive complexes as a function of external compressive forces. To conclude, our work will help to elucidate the framework by which external forces modify intracellular mechanical properties to allow cell shape changes to occur.
In summary, our project will generate fundamental insights into how cell shape develops as a function of the mechanical properties of the environment. We propose a multidisciplinary approach (including 4D reconstructions, homoFRET, biophysics and optogenetics) to achieve our objectives.