Aline Schmitt
Stereoselective recognition of glucopyranosides and chiral neurotransmitters by enantiopure hemicryptophanes
Aline Schmitt
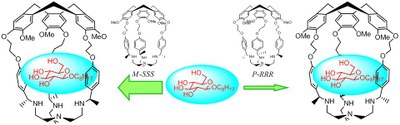
Hemicryptophanes[1] are molecular containers which consist of a chiral cyclotriveratrylene (CTV) moiety connected to an organic group of C3-symmetry with three tentacles whose nature and length can be varied. These heteroditopic host molecules were found to be efficient supramolecular catalysts[2] and molecular receptors for various substrates[3]. Nevertheless, their inherent chirality has rarely been explored. A challenge for these receptors would be to show stereoselective recognitions of enantiopure biological molecules like carbohydrates[4,5] or neurotransmitters[6]. For this purpose, we synthesized eight new enantiopure hemicryptophanes and we determined their absolute configuration by circular dichroism. Then, we studied their complexations with several enantiopure glucopyranosides and neurotransmitters. This work showed enantio-selective and diastereo-selective complexations of the biological molecules by these hemicryptophanes.
[1] J. Canceill, A. Collet, J.Gebard, F. Kotzyba-Hibert, J-M Lehn, Helv. Chim. Act. 1982, 65, 1894-1897.
[2] A. Martinez, J-P. Dutasta, J. Catal. 2009, 267, 188-192.
[3] O. Perraud, V. Robert, H. Gornitzka, A. Martinez, J-P. Dutasta, Angew. Chem. Int. Ed. 2012, 2, 504-508.
[4] O. Perraud, A. Martinez, J-P. Dutasta, Chem. Commun. 2011, 47, 5861-5863.
[5] (a) A. P. Davis, Org. Biomol. Chem., 2009, 7, 3629-3638.
(b) A. P. Davis, Nature, 2010, 464, 169-170.
(c) M. Marzik, Chem. Soc. Rev., 2009, 38, 935-956.
[6] S.-G. Kim, K.-H. Kim, J. Jung, S. K. Shin, K. H. Ahn J. Am. Chem. Soc., 2002, 124 , 591–596.