Jérémy Zaffran
Fast prediction of polyols reactivity at metallic surfaces
Jérémie Zaffran
Biomass transformation is an interesting alternative to fossil resources, which is frequently performed via heterogeneous catalysis. Designing new catalysts and optimizing them is a challenging task that can be significantly accelerated in silico. However, biomass molecules are often complex and highly oxygenated such as sugar or polyols. As a result it is quite difficult and time consuming to study their reactivity using exclusively DFT-based computational tools. Therefore, a method to predict easily and directly the reactivity of such compounds is a necessity.
Using the VASP code and the Brønsted-Evans-Polanyi (BEP) type relationships[1,2,3], we developed a model to predict glycerol reactivity from simple alcohols on metallic catalysts. Such relationships aim at deducing a kinetic value from a thermodynamic parameter, avoiding thus a high computational cost.
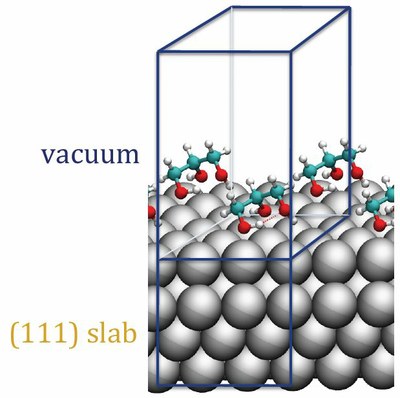
Figure: Glycerol on an Rh (111) surface of Rh.
In Grey: Rh atoms, in green: C atoms, in red: O atoms, in white: H atoms
We focused initially on Rh (111) surface and found that the activation energies for glycerol dehydrogenation are deducible from simple alcohols with an average error on the order of ~10 kJ/mol. Encouraged by this good agreement, we proceeded to establish similar models on other catalytically interesting transition metals (Pt, Pd, Ir, Ni). This method provided also a low error in the prediction of activation energies for simple alcohols dehydrogenation on these other surfaces, and could be applied to more complex molecules.
1-Van Santen, R. A.; Neurock, M.; Shetty, S. G. Chem. Rev. 2010, 110, 2005−2048.
2-Alcala, R.; Mavrikakis, M.; Dumesic, J. A. J. Catal. 2003, 218, 178−190.
3-Loffreda, D.; Delbecq, F.; Vigné, F.; Sautet, P. Angew. Chem., Int. Ed. 2009, 48, 8978−8980.



