Bastien Chatelet
Superbases in the confined space of hemicryptophane hosts: control of the basicity and influence on the reactivity of the proton transfer
Bastien Chatelet
The kinetic and thermodynamic of proton transfer in biological systems can be strongly modified by the surrounding medium. Changes in the reactivity in such confined biological entities have driven chemists to explain and mimic them through the design of supramolecular structures.
The proazaphosphatranes 1, with the related azaphosphatranes [1•H]+Cl– (Figure 1), first synthesized by Verkade et al.,[1] are non-ionic superbases (pKa ≈ 32) now broadly used in organic synthesis as stoichiometric bases and as catalysts.[2]

Figure 1 Structures of azaphosphatrane and proazaphosphatrane derivatives.
Recently, we reported the synthesis of an encaged proazaphosphatrane superbase in a hemicryptophane-type structure, and we investigated the thermodynamic and kinetic consequences of the encapsulation.[3] This study showed that encapsulation does not alter the strong basicity of the proazaphosphatrane, but dramatically decreased the rate of proton transfer. Herein, we wish to demonstrate how the size and the shape of the nanospace around this highly reactive center can control the basicity and the rate of the proton transfer. New encaged azaphosphatranes were synthesized (Figure 2), having a molecular cavity, with different volumes and shapes, above the reactive center. We will see how these tailored-made nanoreactors provide a unique tool to study how confinement can affect the behavior of an encaged active site.
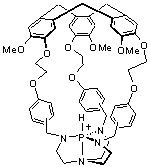
Figure 2 Structure of encaged azaphosphatranes
[1] Lensink, S. K. X.; Verkade, J. G. J. Am. Chem. Soc, 1989, 111, 3478-3479.
[2] (a) Kisanga, P. B.; Verkade, J. G.; Schwesinger, R. J. Org. Chem. 2000, 65, 5431-5432.
(b) Raders, M. R.; Verkade, J. G. J. Org. Chem. 2010, 75, 5308-5311.
(c) Kisanga, P. B.; Verkade, J. G. Tetrahedron, 2001, 57, 467-475.
(d) For a review see: Verkade, J. G.; Kisanga, P. Tetrahedron 2003, 59, 7819-7858 and references therein.
[3] P. Dimitrov-Raytchev, A. Martinez, H. Gornitzka and J.-P. Dutasta, J. Am. Chem. Soc. 2011, 133, 2157-2159.



