Bastien Mettra
Molecular engineering of two photons absorbing chromophores. Towards understanding of the excited state photophysics.
Bastien Mettra
Chromophores with two-photon absorption (TPA) [1] in the biological transparency window (700-1000nm) [2] and an accessible triplet excited state able to generate singlet oxygen (1O2) are good candidate for photodynamic therapy (PDT). TPA allows deeper penetration of light into tissues and better control on the irradiation volume, whereas production of 1O2 occurs through interaction with the chromophore excited triplet state. Our aim is to understand how the quantum yield of 1O2 generation and other photophysical parameters of interest are related to some key structural parameters of the chromophores : bromide substitution pattern and molecule’s symmetry [3,4].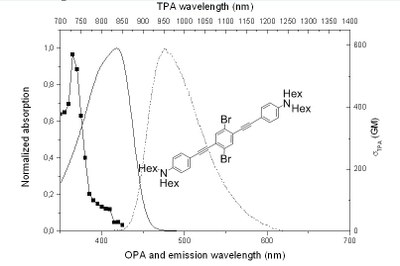

Figure 1.Evolution of the one- (full) and two-photon (full+marks) absorbtion and emission (dotted) properties between two synthesized molecules
We will present the design and the synthesis of new molecules, all belonging to the same family as in Figure 1. For each molecule, the evolution of photophysical characteristics will be investigated. Then we will discuss the relationship between bromide substitution pattern, molecule’s symmetry spectroscopic and photophysical data. Finally, the possibility to use some of these chromophores for biological applications will be discussed.
[1] J. P. Celli, B. Q. Spring, I. Rizvi, C. L. Evans, K. S. Samkoe, S. Verma, B. W. Pogue and T. Hasan, Chem. Rev., 2010, 110, 2795-2838.
[2] E. Skovsen, J. W. Snyder and P. R. Ogilby, Photochem. Photobiol., 2006, 82, 1187-1197.
[3] Gallavardin, T.; Armagnat, C.; Maury, O.; Baldeck, P. L.; Lindgren, M.; Monnereau, C.; Andraud, C. Chem. Comm. 2012, 48, 1689-1691.
[4] Pierre-Henri Lanoë, Thibault Gallavardin, Aurore Dupin, Olivier Maury, Patrice L. Baldeck, Mikael Lindgren, Cyrille Monnereau and Chantal Andraud, Org. Biomol. Chem., 2012, 10, 6275-6278.



