Guillaume Gros
Modular construction of N-CO-interacted inhibitor candidates and their biological assessment with HIV-1 protease
Guillaume Gros
Moieties mimicking the transition-state of peptide hydrolysis have proved efficient in the design of inhibitors for aspartic proteases. [1-3] Recently, we have reported on the synthesis of a hydrazino urea that displays a rarely observed N-C=O interaction when dissolved in polar protic media and shows micromolar inhibition potency toward HIV-1 protease. [4] These results support our hypothesis that the moiety resulting from this interaction may reproduce electrostatic properties of the transition state of peptide hydrolysis. In our search for more potent inhibitors, we can benefit from the modular construction of our targets, as shown below.
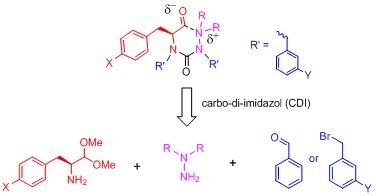
This study describes the synthesis, characterization and biological assays of a range of hydrazino ureas. We are now able to modify selectively either the amino acid-derived building block (in red), the hydrazine moiety or the urea pendant arms. For the first time, we have preparedan exhaustively substituted urea. The inhibitory potency of our previous derivatives is essentially maintained by our new candidates, as determined by two different biological assays.
1. D. H. Rich, J. Med. Chem. 1985, 28, 263.
2. R. Wolfenden, Bioorg. Med. Chem. 1999, 7, 647.
3. J. D. A. Tyndall, T. Nall, D. P. Fairlie, Chem. Rev. 2005, 105, 973-999.
4. M. Waibel, D. Pitrat, J. Hasserodt, Bioorg. Med. Chem. 2009, 17, 3671-3679.



