Martin Ipuy
Synthesis of new pH sensitive fluorescent probes based on the TCF electron accepting group
Martin Ipuy
Phenolic compounds are amongst the most important class of dyes. The main characteristic property of phenols is the acidity of the OH group[1]. The hydroxy group OH is a weak electron donating group whereas the deprotonated O- is a much stronger donating group with a negative charge than can delocalize along a conjugated path conferring different optical properties to the phenol and the corresponding phenolate. This unique property has been intensively exploited in the design of fluorescent indicator for intracellular pH and most of the pH indicators developed so far are phenol derivatives spanning the physiological pH.[2] Functionalization of the hydroxyl group also strongly modifies the optical properties of the original phenol chromophore and removes the acidic hydrogen. As such, phenol fluorophores have often been used in the design of off/on enzyme responsive probes[3, 4].
We will present here the design, the synthesis and the study of the physical and optical properties of a new series of simple push-pull dipolar phenol derivatives containing the 2-dicyanomethylene-3-cyano-4,5,5-trimethyl-2,5-dihydrofurane (TCF) ring. Those fluorophores present a very fast cellular uptake and are easily visualized by fluorescence microscopy making them very good candidates for intracellular pH probes. We will also present the functionalization with enzyme cleavable substrates.
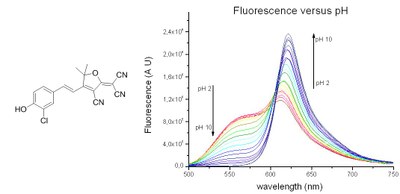
Figure 1. Example of pH sensitive phenol. Left: (E)-2-(4-(3-chloro-4-hydroxystyryl)-TCF. Right: fluorescence spectrum in H2O/DMSO 4/1 with excitation at 490 nm.
[1] M. D. Liptak, K. C. Gross, P. G. Seybold, S. Feldgus and G. C. Shields, J. Am. Chem. Soc. 2002, 124, 6421-6427.
[2] J. Han and K. Burgess, Chem. Rev. 2010, 110, 2709–2728.
[3] K. R. Gee, W.-C. Sun, M. K. Bhalgat, R. H. Upson, D. H. Klaubert, K. A. Latham and R. P. Haugland, Anal. Biochem. 1999, 273, 41-48.
[4] C. W. Lee, Y. H. Song, Y. Lee, K. S. Ryu and K.-W. Chi, Chem. Commun. 2009, 6282-6284.



