Recognition properties of phosphorylated cavitands
Phosphonate cavitands
|
Self-assembly of phosphonate cavitands
|
Phosphorylated cavitands form strong associations with ammonium partners. We used this property to elaborate supramolecular association with original structure and properties. For instance, in this context iii-phosphorylated cavitands incorporating a N-methyl-pyridinium guest moiety as fourth bridging unit form supramolecular associations by inclusion of the charged CH3N+-pyridinium head into a neighboring host cavity. The dimeric association is favored in solution and was characterized by NMR, mass spectrometry, DOSY experiments and single crystal X-ray analysis (J. Org. Chem. 2009). |
 |
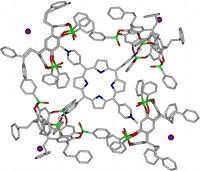 |
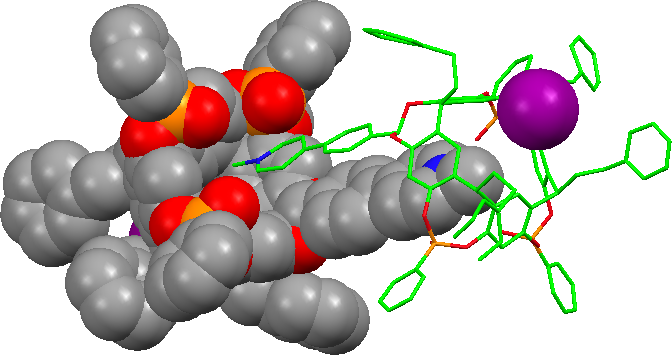
In the same way, four tetra-phosphorylated TiiiiPO cavitands encapsulated the pyridinium heads of a tetra-(N-methylpyridinium)-porphyrin iodide to form a 4:1:4 (host)4/guest4+/4I- complex. The single crystal X-ray diffraction analysis shows the arrangement of the four cavities bound to the CH3N+ groups of the porphyrin moiety and the four iodide anions nested between the phenethyl substituents of the hosts. 1H-NMR investigations show that the structure is preserved in chloroform solution and underline the effect of the counter-anions (J. Org. Chem, 2007). |



