Propriétés de reconaissance des cavitands phosphorylés
Cavitands Phosphonate
Les cavitands phosphonate constituent une nouvelle classe de récepteurs synthétiques pour élaborer des capteurs supramoléculaires. Nous avons développé la chimie de ces hôtes originaux depuis plusieurs années. Au cours de la dernière décennie, nous avons entrepris une collaboration avec le groupe de E. Dalcanale, à Parme (Italie), où sont développés plusieurs types de capteurs pour la détection de différents solutés à analyser. Dans ce contexte, notre expérience dans la synthèse de cavitands phosphorylées nous a permis d'approfondir l'étude de leurs extraordinaires propriétés complexantes. Récemment, les propriétés de reconnaissance moléculaire de cavitands tétraphosphonate envers les alcools et l'eau à l'interface gaz-solide ont été évalués au moyen de trois techniques complémentaires et comparés à celles des cavitands parents mono- et diphosphonate. L'utilisation combinée de la spectroscopie de masse ESI-MS et de la cristallographie par rayons-X a permis de caractériser précisément l'association entre hôte et invité à l'interface en termes de type, de nombre, de force et de géométrie de leurs interactions. Des pesées avec une microbalance à quartz (QCM) ont ensuite validé les valeurs prédites à partir des informations fournies par le capteur. L'importance de multiples interactions énergétiquement équivalentes pour la sélectivité du capteur a été démontrée en comparant les propriétés de reconnaissance moléculaire de cavitands tétraphosphonate avec celles de leurs homologues mono- et diphosphonate (Chem. Eur. J. 2008).
|
Auto-assemblage de cavitands phosphonate
|
Les cavitands phosphorylée s’associent fortement avec les ions ammonium. Nous avons utilisé cette propriété pour élaborer des associations supramoléculaires possédant des structures et des propriétés originales. Par exemple, dans ce contexte, des cavitands iii-phosphorylées incorporant des fragments d'hôtes N-méthylpyridinium en quatrième position d'unité pontante forment des associations supramoléculaires par inclusion de la partie chargée CH3N+ de l’ion pyridinium dans une cavité voisine. Le dimère résultant de cette association se forme facilement en solution. Il a été caractérisé par RMN, traditionnelle et séquence DOSY, par spectrométrie de masse et par rayons-X sur monocristal (J. Org. Chem. 2009).
|
 |
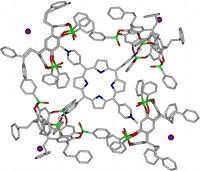 |
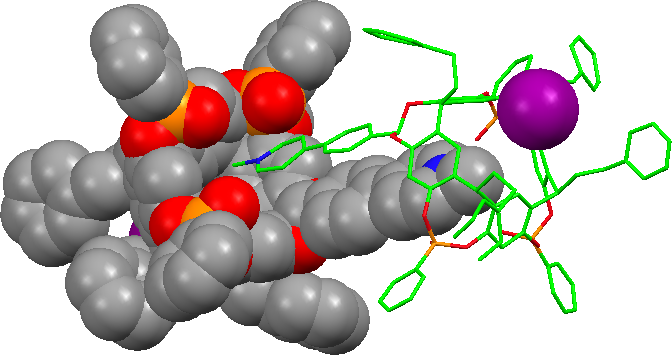
De la même façon, quatre cavitands tétraphosphorylés TiiiiPO ont encapsulé la partie cationique pyridinium d’un iodure de tétra-(N-méthylpyridinium)porphyrine pour former un complexes 4:1:4 (hôte)4/invité4+/4I–. L'analyse par diffraction de rayons-X d’un monocristal montre la disposition des quatre cavités liées aux parties cationiques CH3N+ de la porphyrine et des quatre anions iodure nichés entre les substituants phénéthyles des hôtes. Des analyse 1H-RMN montrent que cette structure est conservée en solution dans le chloroforme et soulignent l'effet des contre-anions (J. Org. Chem, 2007).
|



