Dr. Andreas Goetz
| When |
Nov 09, 2016 à 10:30 AM |
|---|---|
| Where |
Grande salle cbp LR6 |
| Contact |
C. Michel |
Insights into structure and function of a cytochrome c oxidase from computer simulations
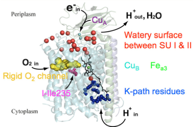
Cytochrome c oxidase (CcO) is the last enzyme in the respiratory electron transport chains of mitochondria and many aerobic bacteria. [1] Its function is to catalyze the reduction of molecular oxygen to water and utilize the resultant energy to pump protons across the inner mitochondrial or bacterial cytoplasmic membrane, forming an electrochemical potential that is utilized by the cell in numerous ways, most prominently for the synthesis of ATP. [2] The catalytic proton pumping cycle of CcO is driven by redox events, molecular oxygen binding, and O2 activation in the dinuclear heme Fea3–CuB complex (DNC). We employ classical molecular dynamics (MD) simulations of the entire enzyme embedded in its membrane and density functional theory (DFT) calculations of large DNC cluster models to explore this redox-coupled proton transport mechanism. [3,4] Our focus is on B-type CcO from Thermus thermophilus, where X-ray structures and high quality spectroscopic and kinetic data are available. In this talk I will highlight DFT results for the energetics of the reaction cycle [4,6] and for Mössbauer parameters that give insight into the spin and coordination states of the intermediates of the reaction cycle. [5] I will also present results from long-time scale classical MD simulations that reveal water exit pathways, which may also act as proton transport pathways. [7]
Acknowledgments
This research is supported by NIH grant GM100934 and computer time via NSF grant ACI-1053575 (award TG-CHE130010).
References
1. Richter, O. M. H.; Ludwig, B. Rev. Physiol. Biochem. Pharmacol. 2003, 147, 47–74.
2. Nicholls, D. G.; Ferguson S. J.; Bioenergetics 3, Academic Press Ltd.: London, 2002.
3. Fee, J. A.; Case, D. A.; Noodleman, L. J. Am. Chem. Soc. 2008, 130, 15002–15021.
4. Noodleman, L.; Han Du, W.; Fee, J. A.; Götz, A. W.; Walker, R. C. Inorg. Chem. 2014, 53, 6458–6472.
5. Han Du, W.; Noodleman, L.; Inorg. Chem. 2015, 54, 7272–7290.
6. Han Du, W.; Götz, A. W.; Yang, L.; Walker, R. C.; Noodleman, L. Phys. Chem. Chem. Phys. 2016, 18, 21162–21171.
7. Yang, L.; Skjevik, A. A.; Han Du, W.; Noodleman, L.; Walker, R. C.; Götz, A. W. BBA, Bioenergetics 2016, 1857, 1594–1606.



