Dr. S. MELISSEN
| When |
Mar 16, 2016 à 10:15 AM |
|---|---|
| Where |
Grande salle cbp LR6 |
| Contact |
T. Lebahers |
Carbon Nitrides - where inorganic, organic and theoretical chemistry meet
The sun is an inexhaustible natural energy source, with about half the solar energy falling within the visible light range. The major drawback of solar energy is its diurnal variation, making its storage in the form of chemical bonds an interesting alternative to photovoltaics. Artificial photosynthesis is one such process, extracting hydrogen gas from water using light.
Graphitic, semiconducting carbon nitrides, with general formula g-CxNyHz, are nowadays integrated as photocaptors in photocatalytic water splitting cells. Several variants are known, including fully polymerized C3N4 and partially polymerized C6N9H3, those based on triazine (t) and those based on heptazine (h). Several highly crystalline species are depicted in the Figure.
Recently, a robust1 computational scheme2 was applied to the four crystalline species in the Figure to predict several key properties of these carbon nitrides.3,4 This revealed that the denser, more cyclicized species were the most promising structures for photocatalytic appli-cations.
At this moment, we are completing a stability study on these compounds.
In this presentation, I will try to bridge experimental and theoretical chemistry. Which particular computational approach is best for which particular problem? What do the obtained results teach us? Finally, we will try to answer the question what role carbon nitrides will have in the future energy mix.4
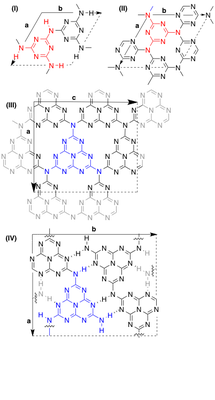
Figure Graphitic carbon nitrides:
(I) gt-C3N4. (II) gt-C6N9H3.
(III) gh-C3N4. (IV) gh-C6N9H3.
References
1. S. Melissen et al., PCCP 17, 2199-2209 (2015)
2. T. Le Bahers et al., J. Chem. Phys. C. 118, 5997–6008 (2014)
3. S. Melissen et al. J. Chem. Phys. C 119, 25188–25196 (2015)
4. M. Bhunia et al. Chem. Mater. 27, 8237–8247 (2015)
5. S. Melissen et al., "Relationship Between Triazine-Based Carbon Nitride Structures and Bandgap: a GW-BSE Perspective" to be submitted to J. Phys. Chem. Lett. (2016)



