Seminar: Harinath Chakrapani
| Quand ? |
16/05/2025 à 10:00 |
|---|---|
| Où ? |
Salle Collet |
| Contact |
Jens Hasserodt |
Abstract
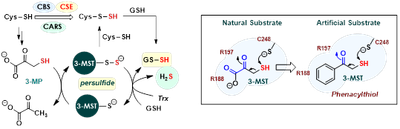
Hydrogen sulfide (H2S), persulfides (RS-SH), and related sulfur species are produced in nearly all cells and have diverse roles, including as antioxidants.1 Cysteine is the primary source of such sulfur species in cells, and enzymes that generate H2S include cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE), cysteinyl-tRNA synthetase (CARS) as well as 3-mercaptopyruvate sulfurtransferase (3-MST).2 With a goal of promoting endogenous antioxidant response, our lab designed and developed artificial substrates for 3-MST.3 The natural substrate for 3-MST is 3-mercaptopyruvate (3-MP), and its turnover produces a persulfide, which can either transfer sulfur to low molecular weight thiols such as glutathione or is cleaved by thioredoxin (Trx) to generate H2S. The first generation of artificial substrates enhanced endogenous persulfides, and protected cells from oxidative stress-induced cell death as well as showed excellent anti-inflammatory properties in animal models.3,4 Using a computational structure-guided approach, we designed and developed a new series of artificial substrates for 3-MST, and we describe results of this approach.4 Together, we provide evidence that supports a new therapeutic paradigm of using small molecules to promote cells’ own antioxidant response.
-
D. Giovinazzo, B. Bursac, J. I. Sbodio, S. Nalluru, T. Vignane, A. M. Snowman, L. M. Albacarys, T. W. Sedlak, R. Torregrossa, M. Whiteman, M. R. Filipovic, S. H. Snyder and B. D. Paul, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2017225118
-
Pedre, B.; Talwar, D.; Barayeu, U.; Schilling, D.; Luzarowski, M.; Sokolowski, M.; Glatt, S.; Dick, T. P. Nat. Chem. Biol. 2023, 19 (4), 507–517.
-
(a) Bora, P.; Manna, S.; Nair, M.; Sathe, R.M.S.; Singh, S.; Adury, V.S.S.; Gupta, K.; Mukherjee, A.; Saini, D. K.; Kamat, S.S.; Hazra, A. B.; Chakrapani, H. “Leveraging an Enzyme/ Artificial Substrate System to Enhance Cellular Persulfides and Mitigate Neuroinflammation” Chem. Sci., 2021, 12, 12939-12949. (b) Manna, S.; Agrawal, R.; Yadav, T. Anand Kumar, T.; Kumari, P. Dalai, A.; Kanade, S. Balasubramanian, N. Singh, A.; Chakrapani, H. “Orthogonal Persulfide Generation through Precision Tools Provides Insights into Mitochondrial Sulfane Sulfur” Angew. Chem. Intl. Ed., 2024, 63, e202411133 (c) Gupta, S. M.; Mohite, P. S. Chakrapani, H. “Mercapto-NSAIDs Generate a Non-Steroidal Anti-Inflammatory Drug (NSAID) and Hydrogen Sulfide” Chem. Sci., 2025, in press
-
(a) Manna, S.; Gupta, S.M; and coworkers, manuscript under review. (b) Gupta, S. M.; and coworkers, manuscript under preparation
|
Pr. Harinath Chakrapani Dept. of Chemistry |
 |
Biography
Harinath Chakrapani completed his undergraduate and post-graduate studies in Chemistry from Loyola College (1994-97) and Indian Institute of Technology Madras (1997-99), respectively. In the fall of 1999, he moved to Duke University, USA to pursue his doctoral studies, which he completed under the supervision of Prof. Eric J. Toone in Dec. 2005. His post-doctoral research work was carried out at Wake Forest University, where he worked with Prof. S. Bruce King and the National Cancer Institute, under the mentorship of Dr. Larry K. Keefer. He joined IISER Pune in July 2009 and is currently Professor.



