Resolution of Hemicryptophanes
The rigidity of the CTV moiety of hemicryptophanes lead to a planar chirality, hence a pair of two enantiomers which can be named by the P and M descriptors. We have achieved the resolution of the racemic mixture and the assignment of the absolute configuration of the CTV unit for each enantiomer of the hemicryptophane derived from tris(N-alkylcarbamoylmethyl)amine. The resolution of the racemic mixture have been succeed thanks to a HPLC semi-preparative method with the following conditions: PIRKLE COVALENT (R,R) WHELK-O 5/100 25 cm x 10,0 mm column, THF:hexane 85:15, 5 ml/min. We obtained a 1.4 separation coefficient which allowed us to isolate a first enantiomer (A) with an enantiomeric excess higher than 99% and a second one (B) with an 96% enantiomeric excess. HPLC spectra (280 nm, respectively for the racemic mixture, A and B) are shown below.

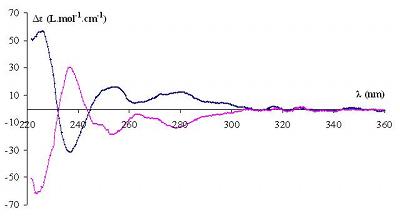
The absolute configurations of A and B were derived from the study of their circular dichroism spectra which have been obtained thanks to a Chirascan apparatus with the following conditions: 20°g;C, c = 0.05 mg.mL-1 in CHCl3. Their symmetry is characteristic of two enantiomers: the first one (A) presents a negative Cotton effect at 232 nm and a positive Cotton effect at 244 nm whereas the second one (B) presents a positive Cotton effect at 232 nm and a negative Cotton effect at 244 nm. These two behaviors are characteristic of hemicryptophane enantiomers and comparison of these spectra to the literature ones allowed us to assign the P configuration to the first enantiomer (A) and the M configuration to the second one (B).