NIR dyes for nonlinear optical applications
We are interested in the design of new dyes featuring both absorption and emission in the NIR spectral range [750-900 nm] and having nonlinear optical properties (two-photon absorption or second-order nonlinear optical properties) for different applications. We are also looking for new strategies to further displace the photophysical properties into the NIR up to 1000 nm.
Polymethyne dyes
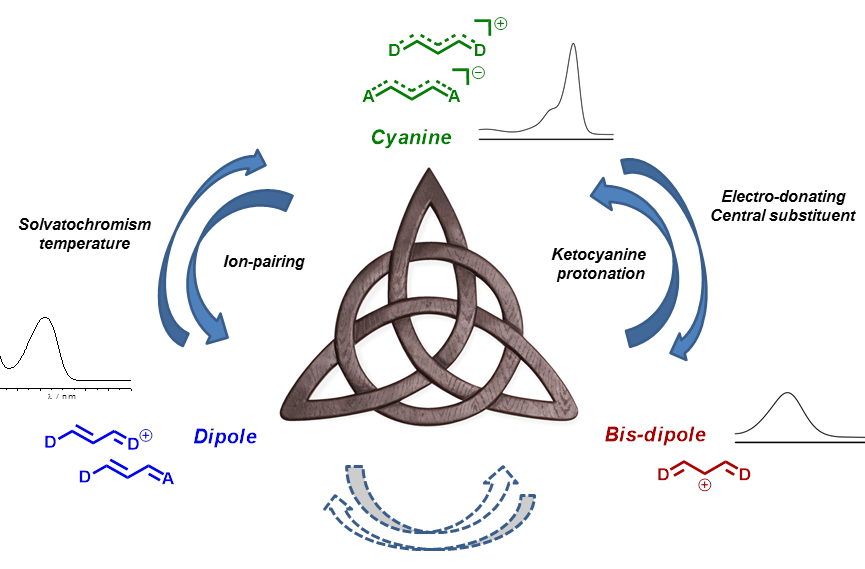
We revisited the spectroscopy of these “old” dyes and demonstrated the existence of three limit electronic configuration namely (i) the fully delocalized cyanine, (ii) the dipole and (iii) the bis-dipole and we studied the different ways to control the charge delocalization between these three states.
Heptamethine cyanine dyes.
In this context we showed that ion pairing effect monitored the cyanine to dipole equilibrium (J. Am. Chem. Soc. 2010, 132, 4328-4335) and that substitution at the central position controlled the cyanine to bis-dipole equilibrium (J. Phys. Chem. A 2014, 118 (23), 4038–4047).
AzaBodipy dyes
In this class of dyes we study the coupling of charge transfer transitions induced by the pheripherical conjugated substituents with the central cyanine type transition localized at the bodipy core both by theoretical and spectroscopic approaches.
Collaborations
Pr. D. Jacquemin (Nantes) and Dr. B. Le Guennic (Rennes) for theoretical simulations (Phys. Chem. Chem. Phys, 2012, 14, 157).
Pr. Perry (Georgiatech, USA) and K. Kamada (Sendai, Japan) for two-photon measurements and ultra-fast spectroscopy (Phys. Chem. Chem. Phys. 2012, 14, 15299).
Pr. E. Kim (Seoul, Korea) Electroswitching of the NIR fluorescence (Chem. Sci., 2014, 5, 1538).



