Publications 2022
Ni-Centered Coordination-Induced Spin-State Switching Triggered by Electrical Stimulation
J.-C. Mulatier, D. Frath, F. Chevallier, C. Bucher et al.
Article available at J. Am. Chem. Soc.
Abstract
We herein report the synthesis and magnetic properties of a Ni(II)-porphyrin tethered to an imidazole ligand through a flexible electron-responsive mechanical hinge. The latter is capable of undergoing a large amplitude and fully reversible folding motion under the effect of electrical stimulation. This redox-triggered movement is exploited to force the axial coordination of the appended imidazole ligand onto the square-planar Ni(II) center, resulting in a change in its spin state from low spin (S = 0) to high spin (S = 1) proceeding with an 80% switching efficiency. The driving force of this reversible folding motion is the π-dimerization between two electrogenerated viologen cation radicals. The folding motion and the associated spin state switching are demonstrated on the grounds of NMR, (spectro)electrochemical, and magnetic data supported by quantum calculations.
Impact of the Syn/Anti Relative Configuration of Cryptophane-222 on the Binding Affinity of Cesium and Thallium
T. Brotinet al.
Article available at ACS Omega
Abstract
We report in this article the synthesis, the X-ray crystal structure of compound syn-2, and its binding properties with cesium and thallium in aqueous solution under basic conditions. Compound syn-2 is the diastereomeric compound of anti-1 that shows very high affinity for cesium and thallium in aqueous solution under the same conditions. Despite the close structural similarities that exist between the syn-2 and anti-1 compounds, they show large discrepancy in their ability to bind cesium and thallium cations in the same conditions. Indeed, the syn-2 derivative has a lower affinity for these two cationic species and the binding constants are several orders of magnitude lower than those found for its congener. The large differences in affinity observed with these two compounds can be explained by the relative position of the six hydroxyl groups to each other.
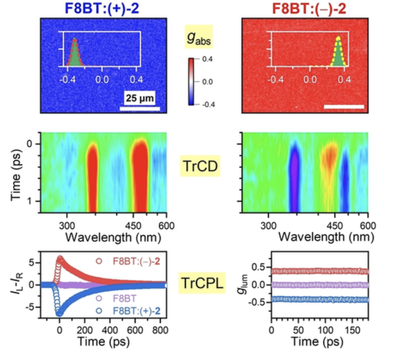
Spatiotemporal Mapping of Efficient Chiral Induction by Helicene-Type Additives in Copolymer Thin Films
L. Guy et al.
Article available at ACIE
Abstract
We observed efficient induction of chirality in polyfluorene copolymer thin films by mixing with helicene-type chiral additives based on the dibenzo[c,h]acridine motif. Images obtained from circular dichroism (CD) and circularly polarized luminescence (CPL) microscopy provide information about the chiral arrangements in the thin films with diffraction-limited resolution. The CD signal shows a characteristic dependence on the film thickness, which supports a supramolecular origin of the strong chiral response of the copolymer. In particular, we demonstrate the discrimination between films of opposite chirality based on their ultrafast transient chiral response through the use of femtosecond broadband CD spectroscopy and a newly developed setup for transient CPL spectroscopy with 28 ps time resolution. A systematic variation of the enantiomeric excess of the chiral additive shows that the “Sergeants and Soldiers” concept and “Majority Rules” are not obeyed.
Elliptical Birefringent Rib-Channel Chirowaveguides for Quasicircularly Polarized Light Applications in Integrated Photonics
L. Guy et al.
Article available at Adv. Photonics Res.
Abstract
Polarization in photonic-integrated circuits (PICs) is governed by transverse electric (TE) and magnetic (TM) polarizations due to the planar structure of the chips. Therefore, all states of polarization (SOPs) other than TE and TM, in particular circular polarization, cannot be routed without modification across the chips. Herein, the realization of rib-channel chirowaveguides supporting elliptically polarized guided modes by combining linear and circular birefringences as predicted by the coupled mode approach is shown. The sol–gel process and the imprint technique to make channel chirowaveguides with different sizes and consequently modulate ellipticity of the eigenmodes from linear to quasicircular are used. Circularly polarized light propagates therefore with a low polarization beating and in particular without handedness inversion. These results open the field of application of PICs to all domains where circularly polarized light is relevant.
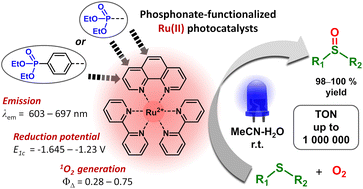
Ruthenium(ii) complexes with phosphonate-substituted phenanthroline ligands: synthesis, characterization and use in organic photocatalysis
C. Bucher et al.
Article available at Dalton Trans.
Abstract
Ru(II) complexes with polypyridyl ligands play a central role in the development of photocatalytic organic reactions. This work is aimed at the structural modification of such complexes to increase their photocatalytic efficiency and adapt them for the preparation of reusable photocatalytic systems. Nine [Ru(phen)(bpy)2]2+-type complexes (bpy = 2,2′-bipyridine, phen = 1,10-phenanthroline) (Ru-Pcat) bearing the P(O)(OEt)2 substituent attached to the phen core directly or through a 1,4-phenylene linker were synthesized and characterized by spectroscopic and electrochemical techniques. The coordination mode of phen ligands was confirmed by single crystal X-ray analysis. The (spectro)electrochemical data show that the first electron transfer in Ru-Pcat takes place on the phen ligand. The emission maxima and quantum yields are strongly affected by the substitution pattern, reaching the far-red region (697 nm) for Ru-3,8P2. The singlet oxygen quantum yields of Ru-Pcat were evaluated using the chemical trapping method. Finally, the photocatalytic performance of Ru-Pcat in the oxidation of sulfides with molecular oxygen was investigated. Both dialkyl and alkyl aryl sulfides were quantitatively transformed into sulfoxides under irradiation with a blue LED in the acetonitrile–water mixture (10 : 1) using a low loading of 0.005–0.05 mol% Ru(II) photocatalysts. To rationalize the effect of phosphonate substituents on the photocatalytic efficiency, comparative kinetic studies of (1) 4-nitrothioanisole oxidation proceeding predominantly via the electron transfer pathway and (2) oxidation of dibutyl sulfide wherein singlet oxygen serves as an oxidant have been performed. It was demonstrated that complexes with the P(O)(OEt)2 substituent at positions 4 and 7 outperform the benchmark photocatalyst Ru-(bpy)3 and the parent complex Ru-phen in the reactions proceeding through electron transfer (reductive quenching photocatalytic cycle). The TON in the oxidation of 4-methoxythioanisole was found to be as high as 1 000 000 that is, to our knowledge, the highest among previously reported photocatalysts. In contrast, upon separating the P(O)(OEt)2 group and the phen core with the 1,4-phenylene linker, singlet oxygen quantum yields significantly increase that favors reactions proceeding through energy transfer (the oxidation of dibutyl sulfide in our case). Thus, both series of Ru(II) complexes prepared in this work are promising for the improvement of known photocatalytic reactions and the development of new transformations.
Oligopyrrolic Cages: From Classic Molecular Constructs to Chemically Responsive Polytopic Receptors
C. Bucher et al.
Article available at Acc. Chem. Res.
Abstract
“Functional molecular systems”, discrete and self-assembled constructs where control over molecular recognition, structure, bonding, transport, release, catalytic activity, etc., is readily achieved, are a topic of current interest. Within this broad paradigm, oligopyrrolic cages have garnered attention due to their responsive recognition features. Due to the presence of slightly polar pyrrole subunits which can also behave as hydrogen-bonding donors, these oligopyrrolic cages are potential receptors for various polarized species. In this Account, we summarize recent advances involving the syntheses and study of (1) covalent oligopyrrolic macrobicyclic cages, (2) oligopyrrolic metallacages, and (3) oligopyrrolic noncovalently linked cages. Considered in concert, these molecular constructs have allowed advances in applied supramolecular chemistry; to date, they have been exploited for selective guest encapsulation studies, anion binding and ion-channel formation, and gas absorption, among other applications. While key findings from others will be noted, in this Account will focus on our own contributions to the chemistry of discrete oligopyrrolic macrocycles and their use in supramolecular host–guest chemistry and sensing applications. In terms of specifics, we will detail how oligopyrrole cages with well-defined molecular geometries permit reversible guest binding under ambient conditions and how the incorporation of pyrrole subunits within larger superstructures allows effective control over anion/conjugate acid binding activity under ambient conditions. We will also provide examples that show how derivatization of these rudimentary macrocyclic cores with various sterically congested β-substituted oligopyrroles can provide entry into more complex supramolecular architectures. In addition, we will detail how hybrid systems that include heterocycles other than pyrrole, such as pyridine and naphthyridine, can be used to create self-assembled materials that show promise as gas-absorbing materials and colorimetric reversible sensors. Studies involving oligopyrrolic polymetallic cages and oligopyrrolic supramolecular cages will also be reviewed. First, we will discuss all-carbon-linked oligopyrrolic bicycles and continue on to present systems linked via amines and imines linkages. Finally, we will summarize recent work on pyrrolic cages created through the use of metal centers or various noncovalent interactions. We hope that this Account will provide researchers with an initial foundation for understanding oligopyrrolic cage chemistry, thereby allowing for further advances in the area. It is expected that the fundamental design and recognition principles made in the area of oligopyrrole cages, as exemplified by our contributions, will be of general use to researchers targeting the design of functional molecular systems. As such, we have structured this Account so as to summarize the past while setting the stage for the future.
Study of syn and anti Xenon-Cryptophanes Complexes Decorated with Aromatic Amine Groups: Chemical Platforms for Accessing New Cryptophanes
M. Doll, T. Brotin, N. De Rycke et al.
Article available at J. Org. Chem.
Abstract
We report the synthesis of C3-symmetric cryptophanes decorated with three aromatic amine groups on the same CTB cap and their interaction with xenon. The relative stereochemistry of these two stereoisomers syn and anti was assessed thanks to the determination of the X-ray structure of an intermediate compound. As previously observed with the tris-aza-cryptophanes analogs anti-1 and syn-2 (J. Org. Chem. 2021, 86, 11, 7648–7658), both compounds anti-5 and syn-6 show a slow in–out exchange dynamics of xenon at 11.7 T. Our work supports the idea that the presence of nitrogen atoms grafted directly onto the cryptophane backbone has a strong impact on the in–out exchange dynamics of xenon whatever their stereochemistry. This result contrasts with the case of other cryptophanes decorated solely with methoxy substituents. Finally, we demonstrate that these new derivatives can be used to design new anti/syn cryptophanes bearing suitable ligands in order to constitute potent 129Xe NMR-based sensors. An example is reported here with the synthesis of the tris-iodo derivatives anti-13 and syn-14 from compounds anti-5 and syn-6.
Photoredox Processes in the Aggregation and Gelation of Electron-Responsive Supramolecular Polymers Based on Viologen
F. Chevallier, C. Bucher et al.
Article available at ECS Adv.
Abstract
Viologen-based ditopic bis-pyridinyl-triazole bidentate ligands self-assemble in the presence of palladium ions into supramolecular polymers whose structure is imposed by the directed formation of coordination bonds. Light-irradiation of these electron-responsive supramolecular materials triggers a photo-induced electron transfer yielding isolated π-radicals and dimers of radicals. The photoreduction events and the associated dimerization steps trigger a large-scale reorganization occurring within the supramolecular network yielding aggregates or gels depending on the irradiation conditions (power, duration). Detailed electrochemical, spectro-electrochemical and photochemical analyses were conducted to understand the mechanisms at stakes in these light-induced aggregation and gelation.
Chiroptical and potential in vitroanti-inflammatory properties of viniferin stereoisomers from grapevine (Vitis vinifera L.)
T. Brotinet al.
Article available at Food Chemistry
Abstract
Determination of stereochemistry and enantiomeric excess in chiral natural molecules is a research of great interest because enantiomers can exhibit different biological activities. Viniferin stilbene dimers are natural molecules present in grape berries and wine but also, in larger amount, in stalks of grapevine. Four stereoisomers of viniferin stilbene dimers (7aS,8aS)-E-ε-viniferin (1a), (7aR,8aR)-E-ε-viniferin (1b), (7aS,8aR)-E-ω-viniferin (2a), and (7aR,8aS)-E-ω-viniferin (2b) were isolated from grapevine stalks of Cabernet Sauvignon, Merlot and Sauvignon Blanc, using a combination of centrifugal partition chromatography (CPC), preparative and chiral HPLC. The structure elucidation of these molecules was achieved by NMR whereas the absolute configurations of the four stereoisomers were investigated by vibrational circular dichroism spectroscopy in combination with density functional theory (DFT) calculations. This study unambiguously established the (+)-(7aS,8aS) and (+)-(7aR,8aS) configurations for E-ε-viniferin and E-ω-viniferin, respectively. Finally, we show that Cabernet Sauvignon provided the quasi enantiopure (+)-(7aS,8aS)-E-ε-viniferin compound which presents the best anti-inflammatory and anti-oxidant activities.
Dedicated to Jean-Pierre Dutasta and Christian Roussel on the occasion of their retirement and to all scientists who contributed to the development of Chirality in France. Historically, the tremendous role played by French scientists in the importance of chirality in chemistry, physics, biology, medicine, or materials science, starting with the pioneering works and discoveries of Augustin Fresnel, Jean-Baptiste Biot, Louis Pasteur, or Aimé Cotton, has been largely acknowledged by the international scientific community. The French chemists and physicists interested by chirality in the broadest sense continued this tradition over the years by being very actively involved in many chirality related research fields such as enantioselective synthesis and catalysis, analytical and chiral separation techniques, chiroptical spectroscopies, theoretical approaches, supramolecular chemistry, polymers, biomimetic systems, chiral materials, and fundamental physics. Witnessing the ever growing interest in France of this interdisciplinary topic, a French network in chirality supported by the CNRS (GDR Chirafun), gathering about 30 chemistry and physics teams from different Institutes, has emerged in 2015, following the organization of the “André Collet Chirality Days” in 2009 and 2012, which were then continued in 2015 and 2018 under the auspices of the Chirafun network, with a new edition planned for October 2022. In this year 2022 marking the bicentenary of Louis Pasteur's birth, we are deeply pleased and honored to introduce this special virtual issue of Chirality gathering 19 very high quality articles spanning different chirality related fields covering
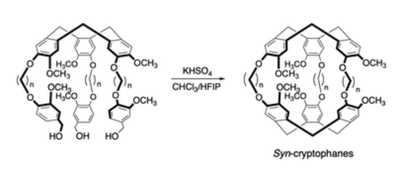
Access to the Syn diastereomers of cryptophane cages using HFIP
J.-P. Dutasta et al.
Article available at Chem. Commun.
Abstract
Cryptophane cages can adopt either an anti or syn configuration that present different recognition properties. While the synthesis of anti-cryptophanes is well reported, the synthesis of syn-cryptophanes remains a challenge. Herein, we demonstrate that the use of HFIP as a co-solvent during the second ring closure reaction significantly affects the regioselectivity, providing easier access to the syn-cryptophane stereomers.
syn-Cryptophanes: macrocyclic compounds with optimized characteristics for the design of 129Xe NMR-based biosensors
T. Brotinet al.
Article available at Phys. Chem. Chem. Phys.
Abstract
A new water-soluble xenon host system with great promise for the 129Xe NMR-based biosensing approach is presented: the syn-cryptophane-222-hexacarboxylate. It compares favorably with its already known antidiastereomer, on the one hand, and with cucurbit[6]uril, on the other hand, in particular in terms of xenon binding constant and xenon in–out exchange, a key parameter for the efficiency of the most sensitive HyperCEST method.
Circularly polarized luminescence of encaged Eu(iii) and Tb(iii) complexes controlled by an inherently chiral remote unit
J.-P. Dutasta et al.
Article available at New J. Chem.
Abstract
A molecular cage-based approach to obtain enantiopure lanthanide complexes with circular polarized luminescence (CPL) activities is presented. Hemicryptophane endohedral complexes were designed by functionalizing the molecular cage with pyridine-2,6-dipicolinamide coordinating groups. Taking advantage of the ability of the inherent chirality of the remote cyclotriveratrylene (CTV) unit to propagate along the linkers of the molecular cage, the encapsulated lanthanide complexes with a controlled chirality have been obtained. In this way, we obtained the CPL active Tb(III) and Eu(III) cages although the chiral CTV unit is located far from the complexation sites. This opens the way for a broader use of enantiopure covalent cages for CPL applications.
New Insights into the Redox Properties of Pyridinium Appended 1,2-Dithienylcyclopentenes
C. Bucher et al.
Article available at ChemPhysChem
Abstract
The optical and redox properties of a methyl pyridinium appended 1,2-dithienylethene photochromic derivative have been thoroughly investigated. A complex multi-step photo/redox mechanism is proposed for the closed isomer on the ground of spectro-electrochemical and theoretical data. The generated compounds are not stable over the time because of chemical reactions associated to the redox processes and a new dithienylethene derivative incorporating a seven-membered ring has been isolated and characterized.
Tuning ON/OFF Ratios in Diarylethene-Based Single- and Bilayer Molecular Junctions
D. Frath et al.
Article available at ECS J. Solid State Sci. Technol.
Abstract
Through electrochemical deposition, photoswitchable single and bilayer molecular junctions based on diarylethene (DAE) and bisthienylbenzene (BTB) layers were fabricated. The electrical characterization of closed and open forms of DAE were investigated by C-AFM for two different layer thicknesses fixed at 2–3 nm and 8–9 nm, i.e. below and above the direct tunneling limit. Both layers switch between high and low conductance modes ("ON" and "OFF" states) when irradiated by UV and visible light. ON/OFF ratios of 2–3 and 200–400 were obtained for 3 nm- and 9 nm-thick DAE MJs, respectively. Next, we prepared 9 nm-thick MJs using a bi-layer system. The first layer (5 nm) is based on BTB oligomers. The second layer (4 nm) is based on DAE oligomers. The impact of this first layer on the switchable properties of the system, and on the photoresponse of the 9 nm-thick DAE-based MJs has been studied. The DAE/BTB bilayer generates new electronic functions combining photoswitching and photorectification. The open form of DAE/BTB shows low conductance and asymmetric I(V)curves while the closed form shows symmetric I(V) curves and high conductance. More importantly, unprecedented ON/OFF current ratios of over 10 000 at 1 volt were reproducibly measured.
Synthesis, Characterizations and Applications of Fluoroazaphosphatranes
J.-P. Dutasta et al.
Article available at Chem. Asian J.
Abstract
Haloazaphosphatranes are the halogenated parents of proazaphosphatranes, also known as Verkade's superbase. While the synthesis of iodo-, bromo- and chloroazaphosphatranes was reported more than thirty years ago by J. G. Verkade, the first synthesis of fluoroazaphosphatranes was only described in 2018 by Stephan et al. Currently, no common and versatile procedure exists to access fluoroazaphosphatranes platform with different structural characteristics. In this report, a new and simple synthesis of this class of compounds was developed based on the nucleophilic attack of the fluoride anion on chloroazaphosphatrane derivatives with good to high isolated yields for the corresponding fluoroazaphosphatranes (70–92%). The scope of the reaction was widened to fluoroazaphosphatranes bearing various substituents and X-ray molecular structures of two of them are reported. The stability of fluoroazaphosphatranes toward nucleophilic solvents like water has been investigated. As they revealed much more robust cations than their chloroazaphosphatrane parents, their chloride salts were tested as organocatalysts for the formation of cyclic carbonates from epoxides and CO2. Fluoroazaphosphatranes proved to be both efficient and stable catalytic systems for CO2 conversion with catalytic activities similar to those of azaphosphatranes, and no decomposition of the cation was observed at the end of reaction.
Frustrated behavior of Lewis/Brønsted pairs inside molecular cages
J.-P. Dutasta et al.
Article available at Org. Chem. Front.
Abstract
Different endohedrally functionalized cages were designed to investigate the effects of the size and shape of molecular cavities on the frustrated behavior of Lewis/Brønsted acid–base pairs and on catalytic activities. The shape of the inner space above the reactive center was found to strongly affect these properties. When an acidic azaphosphatrane is inserted in the smallest cage and associated with t-BuOK or −CD2CN bases, a frustrated Brønsted pair is obtained. In contrast, when encapsulated in the medium or large cage, the P–H+ azaphosphatrane acid is easily deprotonated under the same conditions. The resulting two supramolecular Verkade's superbases lead to frustrated Lewis pair systems in the presence of TiCl4. Furthermore, the larger cage displays better catalytic activity in the MBH reaction. Thus, a small change in the cage size, which only differs by one methylene group in the linkers, can influence the frustrated properties of the related systems, and a right balance between the frustrated behavior and cage flexibility has to be reached to obtain optimal systems for catalytic applications.