Publication of the LCH in the journal Angewandte Chemie International Edition on April 24, 2022. CNRS-INSB communication on May 24, 2022.
A new reagent has been designed through 2-electron activation of SF6 with commercially available tetrakis(dimethylamino)ethylene (TDAE) under blue LED irradiation. The versatility of this new SF5-based reagent has been demonstrated for the deoxyfluorination of CO2 and the fluorinative desulfurization of CS2 affording useful fluorinated amines. Moreover, SF5Cl could be generated under mild conditions from reagent I allowing chloro-pentafluorosulfanylation of alkenes and alkynes.
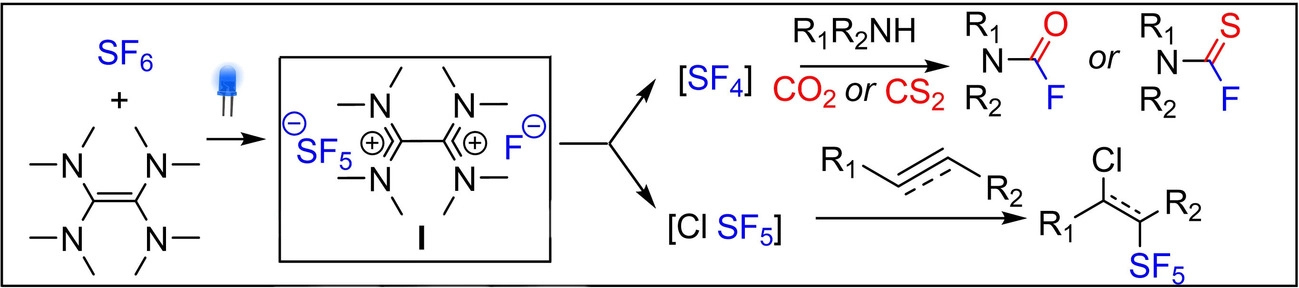
The activation of SF6, a potent greenhouse gas, under metal-free and visible light conditions is reported. Herein, mechanistic investigations including EPR spectroscopy, NMR studies and cyclic voltammetry allowed the rational design of a new fluorinating reagent which was synthesized from the 2-electron activation of SF6 with commercially available TDAE. This new SF5-based reagent was efficiently employed for the deoxyfluorination of CO2 and the fluorinative desulfurization of CS2 allowing the formation of useful fluorinated amines. Moreover, for the first time we demonstrated that our SF5-based reagent could afford the mild generation of Cl-SF5 gas. This finding was exploited for the chloro-pentafluorosulfanylation of alkynes and alkenes.
Credits: Anis Tlili
Reference: Metal-Free SF6 Activation: A New SF5-Based Reagent Enables Deoxyfluorination and Pentafluorosulfanylation Reactions. Alexis Taponard, Tristan Jarrosson, Maurice Médebielle, Lhoussain Khrouz, Julie Broggi and Anis Tlili. Angewandte Chemie International Edition, April 24,2022.
DOI : https://doi.org/10.1002/anie.202204623






